UTOPIAN PHARMACOLOGY
Mental Health in the Third Millennium
MDMA and Beyond
- MDMA/Ecstasy
- A brief history of MDMA
- The MDMA Experience
- MDMA : neurotoxicity
- MDMA : neuroprotection
- Ecstasy for life?
- The molecular machinery of magic
- Post-Darwinian Medicine
- Beyond MDMA : mental superhealth
MDMA/Ecstasy
Can safe, sustainable analogues of MDMA be developed? There is an urgent need for non-neurotoxic empathogens and entactogens suitable for lifelong use. Alas no single "magic bullet" yet exists that replicates the subjective effects of MDMA on a long-term basis. Hence most of us are doomed to display the quasi-psychopathic indifference to each other characteristic of the MDMA-naïve state.A brief history of MDMA
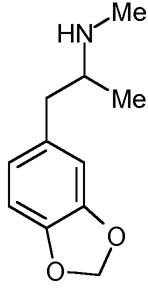
MDMA [3,4-methylenedioxy-methamphetamine: 'Ecstasy'] was first1 synthesized in 1912 by the German pharmaceutical company Merck. MDMA was patented in Darmstadt, Germany on May 16th 1914, issue number 274,350; and promptly forgotten. Merck's researchers had no idea of the significance of what they had done. Merck were searching for a good vasoconstrictor, a styptic to reduce bleeding. In 1912 two of their chemists, G. Mannish and W. Jacobsohn, created MDMA as a by-product while attempting to synthesise hydrastinin. MDMA is listed on Merck's patent-application merely as a chemical intermediate "for products of potential pharmaceutical value".MDMA surfaced again briefly as one of a number of agents used in clandestine US military research during the 1950s. The CIA's Project MK-Ultra was investigating new techniques of brainwashing, espionage and mind-control. MDMA, code-named EA-1475, was tested at the US Army's Edgewood Arsenal in Maryland. However, unlike LSD or the ill-named "truth drug" scopolamine, MDMA was used only on non-human animals: mice, rats, pigs, monkeys and dogs. Thankfully, MDMA's military potential was not realised. For although MDMA is no infallible truth-serum, its effects on the human user might indeed be abused for sinister purposes by skilled interrogators. The heightened emotional responsiveness, lowering of defensive barriers, openness and sense of closeness to others induced by MDMA can promote an honesty of self-disclosure that might be manipulated for malign ends. Fortunately, this hasn't yet happened on an organised scale.
MDMA's parent and longer-acting metabolite, 3,4-methylenedioxyamphetamine [MDA] was first synthesized in 1910 by the same two unsung Merck researchers who went on to create MDMA. MDMA differs structurally from MDA only in its additional methyl group attached to the nitrogen atom. MDA's own empathy-enhancing effect at low doses was explored by Chilean anthropologist-psychiatrist Dr Claudio Naranjo in his private practice. Dr Naranjo discusses MDA-assisted therapy in his classic The Healing Journey (1973). MDA was patented by drug company SmithKline French for use as a tranquilliser (1960) and appetite-inhibitor (1961). SmithKline were interested in MDA's potential as an antidepressant and a slimming-drug. In 1958 human trials were conducted; unfortunately the compound was to prove too psychedelic for licensed clinical use. But MDA was popular as "the love drug" in the counterculture of the 1960s.
The identity of the first human being to take MDMA/Ecstasy isn't known. The drug gained prominence only in the late 1970s. Tipped off by Merrie Kleinman, a graduate student in the medicinal chemistry group he advised at San Francisco State University, the legendary Californian psychedelic chemist Alexander ("Sasha") Shulgin (1925 - 2014) synthesized and taste-tested MDMA at incrementally ascending doses. Ironically, Dr Shulgin had himself synthesized MDMA in 1965, but hadn't tried it, an error of omission he later did much to repair. The effects of a 120mg dose of MDMA are recorded in Dr Shulgin's lab-notes (Sept 1976):
"I feel absolutely clean inside, and there is nothing but pure euphoria. I have never felt so great or believed this to be possible. The cleanliness, clarity, and marvelous feeling of solid inner strength continued throughout the rest of the day and evening. I am overcome by the profundity of the experience..."In the first published scholarly paper [Shulgin,A.T. & Nichols,D.E.: Characterization of three new psychotomimetics. In: Stillman,R.C. & Willette,R.E. (Eds.) The Pharmacology of hallucinogens. New York: Pergamon, 1978] on MDMA use in humans, Dr Shulgin and Dr David Nichols describe the effects of MDMA on the human psyche as "an easily controlled altered state of consciousness with emotional and sensual overtones." The well-connected stepfather of MDMA soon introduced the drug to the wider scientific community. Some of Dr Shulgin's friends, notably the "Johnny Appleseed of MDMA", Leo Zeff, were professional therapists. They in turn introduced MDMA to colleagues as a valuable adjunct to psychotherapy.Later, in 1991, Dr Shulgin and his wife Ann published PiHKAL [Phenethylamines I Have Known And Loved]: A Chemical Love Story. PiHKAL describes the synthesis and systematic testing on human subjects of a range of novel or neglected phenethylamine research drugs. PiHKAL also offers a uniquely sophisticated methodology for human psychopharmacology and the scientific study of mind as an experimental discipline.
By the early 1980s, over a thousand private psychotherapists in the USA were using MDMA in their clinical practice. MDMA was commonly known as "Adam", an allusion to "being returned to the natural state of innocence before guilt, shame and unworthiness arose". MDMA was used discreetly; no one wanted a re-run of the 60s. Dr Shulgin himself reportedly felt MDMA came closest to fulfilling his ambition of finding the perfect psychotherapeutic drug.
Inevitably word leaked out. MDMA was profiled by the San Francisco Chronicle as "The Yuppie Psychedelic" (10 June 1984). In Newsweek, J Adler ["High on 'Ecstasy", April 15 1985] likened his MDMA experience to "a year of therapy in two hours". Harpers Bazaar described MDMA as "the hottest thing in the continuing search for happiness through chemistry". Unsurprisingly, MDMA use soon spread beyond the couch and clinic to the wider world. MDMA's now universal brand-name, "Ecstasy", was coined in 1981 by a member of a Los Angeles distribution network. The unnamed distributor, quoted in Bruce Eisner's Ecstasy:The MDMA Story (1989), apparently chose the name "Ecstasy" because "it would sell better than calling it 'Empathy'. 'Empathy' would be more appropriate, but how many people know what it means?" Condemned by purists as a cynical marketing ploy, the brand-name "Ecstasy" isn't wholly misleading [ecstasy: "an overpowering emotion or exaltation; a state of sudden intense feeling. Rapturous delight. The frenzy of poetic inspiration. Mental transport or rapture from the contemplation of divine things"]. Many first-time MDMA users do indeed become ecstatic. Some people report feeling truly well for the first time in their lives.
In the early 1980s, American production of MDMA beyond the research laboratory was effectively controlled by chemists known as the "Boston Group". Somewhat incongruously, MDMA was especially popular in Texas, where the Southwest distributor for the Boston Group launched his own commercial operation. Mass-production of MDMA by the so-called "Texas Group" began in 1983; supply (and demand) soon mushroomed. Ecstasy was distributed openly in bars and nightclubs in Dallas and Fort Worth. It could be purchased via toll-free 800-numbers by credit card. The drug was even marketed via pyramid-style selling-schemes. Ecstasy could be bought in little bottles at convenience stores under the label "Sassyfras", a tongue-in-cheek allusion to the botanical origins of its precursor.
The DEA reacted by petitioning to have MDMA banned altogether. In 1985 the drug-warriors succeeded in having MDMA made Schedule One. Schedule One is the most restricted of all drug categories i.e. MDMA had allegedly "no legitimate medical use or manufacturer" in the USA; it lacked safety for use even under medical supervision; and it carried a "high potential for abuse". But by then MDMA's fame had spread across the Atlantic. MDMA had metamorphosed from "Adam", the psychotherapeutic tool, to "Ecstasy", the party drug.
MDMA was first introduced to Europe via the sannyasins, disciples of the Bhagwan Shree Rajneesh. "Sannyasa" is a Sanskrit word meaning complete or perfect renunciation. Cult members slipped MDMA into the drinks of rich sympathisers to open up their hearts and their wallets.
Ecstasy became associated with the birth of Acid House music in the Spanish tourist resort of Ibiza. By the summer of '86, Ibiza was popularly known as "XTC Island". Returning tourists and disc-jockeys took the message back home. The UK's rave scene was born. Hundreds of thousands of tablets were consumed each weekend in the famous "Summer of Love" (1988). The Conservative Government and its allies in the British press were aghast. A moral panic set in at the threat to the nation's youth. MDA, MDEA, MDMA and assorted psychedelic amphetamines had been outlawed in the UK since 1977. Yet the Criminal Justice and Public Order Act 1994 sought to criminalize an entire youth-culture by suppressing music played publicly with "sounds wholly or predominantly characterised by the emission of a succession of repetitive beats".
Soon production and distribution of the world's leading empathogen-entactogen fell into the hands of organised crime. By the turn of the millennium, perhaps 80-90% of the world's MDMA was manufactured in Belgium and the Netherlands. Russian-Israeli syndicates and Eastern European chemists are now increasingly active too. The expertise needed in MDMA production varies according to the route of synthesis. Over twenty recipes have been described in the literature. Only seven are common. Clandestine production is easiest starting with MDP2P. MDP2P (3,4-methylenedioxyphenyl-2-propanone) is a commercial product used by the flavouring and fragrance industry. Groups with access to MDP2P can make MDMA via a simple conversion process. Otherwise, MDMA must be synthesized from piperonal, isosafrole, or safrole. These primary precursor chemicals of MDMA are produced in India, China, Poland, Germany, and increasingly elsewhere. Typically, safrole or isosafrole are first converted to MDP2P. The essential oil safrole occurs naturally as the primary constituent of oil of sassafras. Oil of sassafras is found in the root-bark of US East Coast tree Sassafras albidum and from the above-ground woody parts of the South American tree Ocotea pretiosa. Safrole is also present in nutmeg (Myristica fragrans), dill, parsley seed, crocus, saffron, vanilla beans, and calamus. If MDMA were on-patent, then today it might be marketed as "natural" or "naturally-inspired"; but Nature has not been so kind.
Early in the twenty-first century, an estimated several million people worldwide were taking Ecstasy and allied research chemicals each month on college campuses, in high schools and on dance-floors. Purity varies; perhaps 10%-15% of tablets consumed contain MDMA as the sole active ingredient. Illicit knowledge of the "penicillin of the soul" is spreading rapidly around the world, but in corrupt and contaminated form.
The MDMA Experience
Pure MDMA salt is a white crystalline solid. It looks white and tastes bitter. The compound is chemically stable. MDMA does not readily decompose in heat, air or light. The optimal adult dose of racemic MDMA is probably around 120-130mg [around 2mg/kg of body weight i.e. about 125mg] but optimal dose ranges from perhaps 75mg to as much as 250mg. Pills sold in clubs often contain less. There are gender differences in response; proportionately to body-weight, women are normally more sensitive than men to the sub-acute and longer-term effects of MDMA, so their optimal dosage may be lower. The preferentially metabolised (+)-enantiomer ("mirror image") of MDMA is more active, more stimulating, more dopaminergic, more subjectively rewarding, and more neurotoxic than the (-)-enantiomer. MDMA is usually taken orally as a tablet, a capsule, or a powder. MDMA is readily absorbed from the gastrointestinal tract into the bloodstream. More rarely, the drug is snorted, smoked or injected.Onset of action is normally within twenty to sixty minutes or so after administration. When MDMA is administered by the oral route, "coming up" is naturally faster on an empty stomach. Taking MDMA causes both an increased neuronal reuptake inhibition of the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) and also, critically, its increased synaptic release. The MDMA molecule is small enough to be taken up via the membrane-bound serotonin transporter into the presynaptic serotonin axon terminals. Here MDMA acts to reverse the normal direction of the so-called serotonin reuptake pump. Inside the nerve cell, MDMA alters the configuration of the transporter protein so it binds to cytoplasmic serotonin, after which the transporter dumps serotonin outside the cell, reversing the normal inward-bound direction of the transporter channel i.e. MDMA increases the rate of transporter-mediated serotonin outflow. The consequent additional flood of serotonin in the user's synapses is soon followed by an increased release of dopamine especially in the reward centres of the striatum and nucleus accumbens. Release of oxytocin, the "cuddle hormone", surges too via stimulation of the serotonin 5-HT(1A) receptors.
First-time MDMA users occasionally feel confused or anxious before the dose-dependent dopamine-release kicks in. A transient hint of nausea is common when coming up. Most of the body's serotonin is found outside the brain, notably in neurons of the enteric nervous system, our "little brain" inside the smooth muscles of the gut. The user's peak experience or plateau phase after the exhilarating dopaminergic "rush" doesn't last much more than ninety minutes to two hours. MDMA's primary effects wear off after some 3-4 hours. MDMA is more fat-soluble than its structural parent, so its speed of onset is slightly faster and its duration of action shorter. With oral MDMA dosing, peak concentration in the plasma follows after around two hours. Therapists then sometimes add(ed) a final 50mg booster-dose. Heavy recreational users are not always so restrained either in dosage ["stacking"] or top-up schedule ["piggybacking"].
The clarity and unique psychological effects of MDMA can be impaired by ethyl alcohol. Thus MDMA is best taken while completely sober, though a modest drink later to ease any comedown may be useful.
MDMA has a complex nonlinear pharmacokinetics. Taking higher and/or more frequent doses of the drug disproportionately increases levels of plasma MDMA. Higher levels substantially increase oxidative stress and magnify the risk of toxicity. MDMA is metabolised via N-demethylation to the active metabolite MDA; MDA can itself induce a state of sensual euphoria, though in humans the conversion rate from MDMA in the body is low. At least four other metabolites have been identified. MDMA is broken down mainly in the liver, primarily by the polymorphic cytochrome P450 enzyme CYP2D6. However, other enzymes are involved in its degradation beside CYP2D6; some of them, like CYP2D6 itself, are saturated at relatively low MDMA concentrations. MDMA metabolism seems to run up against such a metabolic saturation-point somewhere between 120 and 150mg. When the high-affinity enzymes are saturated, a disproportionately large increase in blood- and brain MDMA-concentrations may occur if the user then takes more of the drug. A large but variable quantity of the parent compound is excreted unchanged, especially when the drug is taken at higher doses; but the opportunities for MDMA recycling by the cost-conscious are normally wasted.
MDMA is sometimes described as a cross between a psychostimulant and a mild hallucinogen. Since it's a methoxylated amphetamine, MDMA is indeed structurally related to mescaline. MDMA's methylenedioxy (O-CH2-O-) group is attached to positions 3 and 4 of the aromatic ring of the amphetamine molecule. But hallucinations on MDMA taken at therapeutic dosages are extremely rare; and psychostimulants, unlike MDMA, don't typically induce a profound sense of inner peace. Thus MDMA exhibits a different profile both from the prototypical "serotonergic" 2,5-dimethoxy-4-methylamphetime (DOM), with its psychedelic 5-HT2A-mediated mechanism of action, and also from the prototypical "dopaminergic" stimulant (+)-amphetamine.
MDMA is perhaps best characterised as belonging to a functionally unique class of "empathogen-entactogen". These words don't mean a great deal in the MDMA-naïve state. The term "empathogen" to describe MDMA and other closely related phenethylamine "empathy drugs" [MDA, MDEA, MBDB] was proposed by Ralph Metzner, Dean of the California Institute of Integral Studies, at a 1983 conference at the University of California at Santa Barbara. The term "entactogen" was coined in 1986 by Dr David Nichols, Professor of Medicinal Chemistry and Pharmacology at Purdue University and co-founder of the Heffter Research Institute, to refer to substances that generate a sense of "touching within" or "produce a feeling in one's innermost being". Both terms are quite apt, though neither will win any marketing awards. MDMA can promote an extraordinary clarity of introspective self-insight, together with a deep love of self and a no less emotionally intense empathetic love of others. MDMA also acts as a euphoriant. The euphoria is usually gentle and subtle; but sometimes profound.
Culture, set and setting inevitably shape the MDMA experience. Idiosyncratic responses to MDMA aren't rare. MDMA has even been described as a drug that "could be all things to all people" (Dr Shulgin). Even so, MDMA's primary effects on the user are surprisingly consistent, unlike the wilder psychedelics such as LSD, psilocybin, or DMT. MDMA may feel mystical, magical or sublime; but it doesn't feel weird. The drug's influence feels highly controllable. MDMA tends to enrich the user's sense of self-identity, not diminish it. MDMA "provides a centering experience, rather than an ego diffusing experience" (Dr. Philip Wolfson), though it may also cause a "softening of the ego-boundaries". Sometimes a degree of derealisation on MDMA may occur, but rarely depersonalisation in the ordinary sense of the term. On the contrary, users feel they can introspectively "touch inside" to their ideal authentic self with total emotional self-honesty.
As well as acting as a "gateway to the soul", MDMA "opens up the heart". Taking MDMA induces an amazing feeling of closeness and connectedness to one's fellow human beings. MDMA triggers intense emotional release beyond the bounds of everyday experience. The drug also enhances the felt intensity of the senses - most exquisitely perhaps the sense of touch. The body-image looks and feels wonderful. Other people look and feel wonderful too. Minutes after dropping a pill, a lifetime of Judaeo-Christian guilt, shame or disgust at the flesh melt away to oblivion.
When MDMA is taken outdoors, the natural world seems vibrant and awe-inspiring, perhaps even enchanted. The experience of colour is gorgeously intensified. On MDMA, Dr Shulgin reported how mountains he'd observed many times before appeared to be so beautiful that he could barely stand looking at them. MDMA is not normally classed as an entheogen. "Entheogen" is a term proposed in 1979 by the scholars R. Gordon Wasson, Carl A.P. Ruck, Jonathan Ott, Jeremy Bigwood and Danny Staples for agents "generating the god or the divine within", shorn of any speculative metaphysics. Yet MDMA is used by a variety of spiritual practitioners of widely diverse beliefs as a gateway to the divine. Some MDMA users undergo life-changing spiritual experiences. Nicholas Saunders, author of the book E for Ecstasy (1993), cites a Benedictine monk who finds MDMA "opens up a direct channel to God". MDMA may not be "Christ in (al)chemical form", but if it had been present in the Eucharist, then we would all still be devout Christians, possibly for ever. A minority of first-time MDMA users undergo what the inventor of the Shulgin scale christened a Plus Four...
"PLUS FOUR, n. (++++) A rare and precious transcendental state, which has been called a "peak experience," a "religious experience," "divine transformation," a "state of Samadhi" and many other names in other cultures. It is not connected to the +1, +2 and +3 of the measuring of a drug's intensity. It is a state of bliss, a participation mystique, a connectedness with both the interior and exterior universes, which has come about after the ingestion of a psychedelic drug, but which is not necessarily repeatable with a subsequent ingestion of the same drug. If a drug (or technique or process) were ever to be discovered which would consistently produce a plus four experience in all human beings, it is conceivable that it would signal the ultimate evolution, and perhaps the end of, the human experiment. (PiHKAL, pages 964-965)"Plus Fours are rare, today. But on MDMA, even the most jaded and world-weary soul with a tin-ear for poetry may "see a world in a grain of sand, And a heaven in a wild flower, Hold infinity in the palm of your hand, And eternity in an hour."MDMA is sensuous and sensual in its effects without being distinctively pro-sexual. Although once dubbed "lover's speed", MDMA is proverbially more of a hugdrug than a lovedrug: "I kissed someone I was in love with and almost felt as if I was going to pass out from the intensity", recalls one American clubber. However, MDMA's capacity to dissolve a lifetime's social inhibitions, prudery and sexual hang-ups means that lovemaking while under its spell is not uncommon. Superfluous clothes tend to get shed. In men, orgasm is more intense than normal but delayed: MDMA retains a residual sympathomimetic activity, triggering a detumescence of the male organ. To ease MDMA-induced performance difficulties, flagging Romeos increasingly combine Ecstasy with Viagra ('Sexstasy'). Unless carefully premeditated, this is not a recipe for safe sex. MDMA may sometimes cause "inappropriate bonding". Prudence should be exercised before taking it with ex-girlfriends, boyfriends or culturally inappropriate love-objects. The effects of MDMA on bonobos ("pygmy chimpanzees"), our sexually uninhibited primate cousins, are unknown.
On pure MDMA, subjects feel at peace with themselves and the world. They discover an enhanced sense of self-worth, self-forgiveness and complete self-acceptance. Cynical thoughts and negative feelings disappear. Aspects of life normally too sensitive to talk about can be explored freely. Heightened feeling allows long-forgotten and repressed emotional memories from childhood to be retrieved with unusual ease. In some settings, painful, highly-charged and even hitherto unmentionable problems may be discussed with (rose-tinted) candour. On MDMA, a lifetime of accumulated psychological barriers and defence-mechanisms go down, somehow magicked out of existence with a pill. Anger, irritability and ingrained fear dissolve; the hostile amygdala is subdued, if only for a few hours. Ecstasy users tell each other affectionately what beautiful people they are; and they do so from the depths of their hearts.
Before the Orwellian-sounding Drug Enforcement Administration [DEA] placed MDMA on Schedule 1 of controlled substances, professional therapists in the USA found MDMA a valuable tool for counselling and marriage-guidance sessions. MDMA's capacity to induce empathetic bliss, heightened introspection and an increased ability and desire to communicate feelings can create a rapport with the therapist and accelerate a successful outcome. MDMA acts to boost self-esteem and self-confidence, while paradoxically diminishing egotism. The user's sense of social isolation vanishes. "I love the world and the world loves me", affirmed one beneficiary of MDMA-assisted therapy.
On a more sceptical note, it's hard scientifically to validate claims of long-lasting therapeutic success. For MDMA's stunning short-term results make double-blind, placebo-controlled trials effectively impossible. Such a problem doesn't always bedevil today's lame "antidepressants", the results of whose trials often struggle to reach statistical significance. Investigational drugs are lab-tested by Big Pharma to discover whether or not non-human animals will self-administer them. Candidate compounds are normally discarded if the animals do so, arguably a perverse route to uncovering antidepressants with good clinical efficacy and high patient compliance. By contrast, MDMA is a warm, fast-acting, non-sedating mood-enricher that banishes social anxiety and physical pain alike. Unlike opioids or the anxiolytic benzodiazepines, MDMA doesn't cloud consciousness even at relatively high doses. This doesn't stop less cerebrally-inclined ravers from getting "cabbaged" by swallowing pills all weekend.
Explored in a controlled setting, MDMA can be therapeutic for victims of Post-Traumatic Stress Disorder (PTSD). A minority of subjects find they enjoy the experience too much to focus on the emotional baggage of the past. Sessions are most likely to be productive with an experienced MDMA therapist. In the Prohibitionist era, MDMA-assisted therapy-sessions are rare.
Dr David Nichols suspects that the related phenethylamine entactogen MBDB ("Eden": 2-Methylamino-1-(3,4-Methylenedioxyphenyl)Butane), formed by extending the 3-carbon chain of MDMA to a 4-carbon chain, might prove superior to MDMA as an adjunct to psychotherapy. This is because Dr Nichols' creation lacks significant dopaminergic activity. It's thus less likely to induce a distracting euphoria. On the other hand, if and when the substrates of blissful self-insight can be sustained indefinitely, then who'll need therapy? Perhaps some inner demons are better left to die of neglect, not awakened for exorcism. Either way, a case can be made that MBDB is indeed a "purer" entactogen than MDMA. Yet as an empathogen, MDMA is unsurpassed and possibly unmatched. MDMA's residual dopaminergic amphetamine-like action contributes a euphoric warmth to the user's intensified feelings and also the desire and ability to express them freely. MBDB's chemical cousin beta-keto-MBDB (bk-MBDB, "Butylone") is a more enjoyable and stimulating empathogen than MBDB. As of 2014, its human use is still extremely limited.
Against formidable odds, the Multidisciplinary Association for Psychedelic Studies (MAPS) has been seeking funding and FDA-approval for controlled trials of MDMA-assisted therapy for PTSD. If these trials are successful, then MAPS hopes that MDMA could eventually become a prescription-medicine. For on MDMA, many traumatized or seemingly emotionally frigid people who can never otherwise speak about their innermost fears and feelings find they can spontaneously open up. There is no compulsion to talk - just a dissipation of the social anxieties that make us normally tight-lipped.
Functional analogues of MDMA may one day be employed in other kinds of insight-oriented therapy as well. Safe, long-acting MDMA analogues may prove therapeutic in the treatment of social phobia, eating disorders and obsessive-compulsive disorder (OCD).
In December 2004, the FDA granted permission for Dr John Halpern's proposed study of MDMA-assisted psychotherapy for patients diagnosed with severe anxiety related to advanced cancer. The likelihood of DEA approval of the protocol is unknown. If the magic of MDMA could be replicated safely and sustainably, then the fear of death and dying could in principle be banished in the population at large. This would be a substantial payoff, though the fear of personal mortality is probably the prime mover of scientific progress in anti-aging research.
Dr Julie Holland, editor of the invaluable Ecstasy:The Complete Guide (2001), tentatively endorses "the judicious, supervised and single oral doses of MDMA as a psychiatric medicine..." In her introduction to the guide, Dr Holland notes that "Like any powerful tool, it should be used by people who are properly trained, educated and supervised. And like any powerful tool, it should come with an instruction manual. This book, I hope, will serve as that manual". It may be testimony to the comparative safety of MDMA that millions of young people use MDMA in the absence of a manual or any training, education and supervision at all. Alas Prohibitionism puts the young and vulnerable at unnecessary risk; and squanders the therapeutic opportunities. In defiance of scepticism from medical orthodoxy, Dr Holland also provides supporting evidence to back up anecdotal reports that MDMA can induce temporary remission of symptoms in victims of otherwise intractable schizophrenia. Less controversially, it's possible for victims of body dysmorphic disorder (BDD), or simply anyone with a negative body self-image, to view themselves in the mirror while euphorically loved-up on MDMA. The transformation can be magical, though it would be imprudent to repeat the experiment two days later.
MDMA can also be used just to have fun. Most commonly today, teenagers and young adults take Ecstasy to rave. Mozart sounds great on Ecstasy, but high-energy all-night dance parties celebrated with techno-pop house music are more standard. Raves are held in clubs, warehouses or more exotic outdoor settings and open fields. Often raves last a whole weekend. The music may be techno, hardcore, jungle, trance or form an improvised, eclectic mix of styles harder to categorise. The atmosphere is astonishingly friendly, the mood and ethos is well captured by the ravers' motto P.L.U.R. ["Peace, Love, Understanding and Respect"]. In darkened clubs, the intoxicating atmosphere of the rave is enhanced with artificial fog, lasers, strobe lights, glow sticks, whistles and Vicks inhalers [on MDMA, aromas are fragrantly enriched]. In many cases, the product now passed off as "Ecstasy" is adulterated with other agents. Individual pills bought by the end-user typically cost between US$7 and US$25. The worldwide street price is falling. Tablets can be mass-manufactured for as little as 50 cents. Professionally-made tablets of MDMA are stamped with distinctive logos. This is because MDMA manufacturers and merchants seek to promote brand-awareness and customer loyalty. Alas counterfeit goods are still rife.
Sometimes "Ecstasy" doesn't contain MDMA at all, but MDA; MDEA (3,4-methylenedioxyethylamphetamine: "Eve"); 2C-B (4-Bromo-2,5 Dimethoxyphenethylamine: ''Nexus", "Venus", "Bromo"); 2C-I; PMA (paramethoxyamphetamine); amphetamine ("speed"); ephedrine; pseudoephedrine; caffeine; the dissociative anaesthetic ketamine ("Special K"); DXM (dextromethorphan); GHB (gamma-hydroxybutyrate: "liquid ecstasy"); or some combination thereof. This list is far from exhaustive. A minority of psychologically robust or reckless clubbers purposely mix MDMA with LSD ("candyflipping") to impart a "warm, loving glow" to their acid trips. Or they "hippieflip" with psilocybin mushrooms; or "kittyflip" with ketamine. Cannabis is widely smoked as well. Ravers who want to dance all night may prefer Ecstasy laced with speed; a sub-neurotoxic dose of MDMA can be made toxic by adding (+)-amphetamine. To outsiders, Ecstasy-fuelled raving might seem mindless hedonism; its devotees have likened it to group-therapy or meditation. But either way, chronic heavy use of the methoxylated amphetamines or any other "club-drug" poses risks to the user's health.
MDMA: neurotoxicity
No compelling evidence exists that taking a single c.125mg dose of MDMA a few times or so a year is likely to cause any long-term harm to the user's mental or physical health. Nevertheless, even pharmaceutical-grade MDMA taken at moderate doses in optimal conditions is not a wholly benign drug. The problem isn't (just) the toxic adulterants used by dance-floor pharmacologists or the botched syntheses of bathtub chemists. Deceptively, and in contrast to most other recreationally used drugs, ingesting pure MDMA can sometimes leave the user feeling better than normal the next day, albeit tired and slightly spaced-out. Beyond warm memories, this afterglow may in part be explained by MDMA's residual amphetamine metabolic by-products: MDMA itself has a long, c.8-9 hour elimination half-life from the blood; and its main metabolite's longer-acting, less stimulating (-)-MDA enantiomer has 5-HT2A activating effects resembling low-grade LSD. But two days or so after taking MDMA, most users experience the serotonin dip. The dip ranges from the almost imperceptible to the markedly unpleasant. The functional deficit the dip reflects may last ten days or more - in some cases possibly weeks or months. A biphasic post-E serotonin profile in the user has been reported: users' serotonin levels - though hard to measure and interpret - apparently fall 3-6 hours after taking the drug, then recover to nearly normal levels after around 24 hours, and then decline again.
Excessive MDMA intake triggers oxidative damage to the user's serotonergic nerve cell fine axon terminal lipids and proteins via the production of toxic free radicals. However, the threshold dose for any lasting MDMA-induced toxicity is unknown; and the identity and precise mechanism of the chemical(s) causing the oxidative stress is unclear. The issue is also controversial. Currently the three leading candidates for guilty agent are:
1] toxic metabolites of MDMAAn excellent review of the published scientific evidence on neurotoxicity is offered by Matthew Baggott and John Mendelson on the indispensable Erowid. A role has also been proposed for nitric oxide; increased Ca2(+); and a toxic intraneuronal metabolite of serotonin. Elevation of body temperature can seriously worsen possible MDMA-induced toxicity; and the thermogenic effect of MDMA is magnified in a hot environment like an indoor rave. Certainly, hypothermia-inducing agents are (partially) neuroprotective against Ecstasy damage; and the primary role of dopamine in MDMA-induced toxicity may actually be to elevate body temperature via its increased action on the dopamine D1 receptors rather than its uptake into the depleted serotonergic axon terminals. But consensus on the molecular mechanisms behind MDMA megadose-induced damage remains elusive.2] toxic metabolites of dopamine
3] impaired cellular energetics
MDMA itself (probably) isn't the culprit. Experimental microinjection of MDMA, MDA or other amphetamine analogues directly into the cerebrum doesn't produce the toxicity to the serotonergic axons ascending from the dorsal raphé nucleus that follows high and/or frequent doses of the peripherally administered drug. MDMA can be centrally injected to induce the release of just as much serotonin as the toxic peripherally-administered dose; but there's still no sign of neurotoxicity. Nor does experimental central MDMA perfusion trigger the toxicity-enhancing higher body temperatures likely from the peripheral route. When MDMA is centrally administered in animal experiments, not even artificially inducing hyperthermia in the victim is enough to produce serotonergic damage. If systemic metabolism of MDMA is indeed necessary for neurotoxicity, the nature of any such possible toxic metabolite(s) is unknown: thioether conjugates of alpha-methyl dopamine have been mooted; and in 2009 neurotoxic thioether adducts of MDMA were detected in humans. Since drug metabolites are normally more hydrophilic than their parent drug, specific transporters are presumably needed to take up the neurotoxic metabolite into the brain; but their identity or even existence isn't known either. If they do exist, then presumably they are monoamines; otherwise selegiline wouldn't be protective against MDMA-induced neurotoxicity.
Whatever the mechanism at work, most users eventually stop taking MDMA. They do so after either they find the E-magic wears off, or the unwanted side-effects of heavy E-use begin to outweigh its joys. Doctors report that one Englishman consumed an estimated 40,000 tablets of MDMA over a nine year period. Such cases are exceptional. Even so, some heavy MDMA users claim they don't experience any long-term adverse effects. Prolonged MDMA administration can even cause a long-lasting increase in the dopamine content of the nucleus accumbens, possibly indicating its disinhibition from normal serotonergic control. The persistent elevation of dopamine function reported in the nucleus accumbens of some MDMA veterans might otherwise be expected to enhance mood, not darken it. Likewise, MDMA users may be less anxious or panic-stricken in response to the normally anxiogenic challenge of a 5-HT2C agonist such as m-chlorophenylpiperazine (m-CPP). Depending on one's ideological agenda, this diminished response to m-CPP can be described as evidence either of serotonergic "toxicity", or alternatively as a pointer to the substrate of a long-lasting "therapeutic" effect. Again, MDMA use increases sensitisation to the rewarding effects of euphoriant dopaminergics such as cocaine; and once more, this is not inherently a sign of "brain damage". However, reports of real and serious health problems from excess E-use are not all prohibitionist propaganda or part of a government-inspired conspiracy to stop young people having a good time. Among heavy "recreational" MDMA users, self-medicating or otherwise, the incidence of depression seems to be more common than healed minds or any enduring therapeutic benefit. The prospect of serotonergic axon terminal degeneration doesn't sound much fun, even if the axons re-sprout - one way or another. Worryingly, the MDMA-induced pruning of the serotonergic axon tree seen at high-dosage regimens leads to altered patterns of reinnervation by ascending axons projecting especially to forebrain sites. In the process of recovery from a prolonged MDMA-binge, the hippocampus, a brain structure critical for episodic memory formation, may actually be hyperinnervated, but reinnervation of the dorsal cortex is sparser. It has been suggested that the heavy MDMA user who discerns no long-lasting ill effects, and who displays minimal functional impairment, may still be subtly damaging his or her serotonergic "functional reserve". The disturbing parallel drawn here is with neurodegenerative disorders: clinical signs of Parkinson's disease, a progressive disorder caused by outright dopaminergic cell death and frequently prefigured by depression, only become apparent after 70-80% of dopamine cells have been lost. It is fiendishly hard to demonstrate MDMA-induced dopaminergic cell damage without virtually killing the victim; in contrived circumstances it can be done. Yet the most notorious attempt to show MDMA-induced dopaminergic neurotoxicity, Ricaurte's September 2002 paper Severe Dopaminergic Neurotoxicity in Primates After a Common Recreational Dose Regimen of MDMA ("Ecstasy") in Science, actually demonstrated methamphetamine-induced dopaminergic neurotoxicity instead. This unfortunate study, its publication timed to coincide with debate in US Congress over the "Anti-Rave Act", was retracted in September 2003; but the spectre it raised of a post-E generation of Parkinsonian zombies may prove harder to dispel.
Not even heroic doses of MDMA are likely to kill off serotonergic brain cells, though there have been unconfirmed reports of MDMA-induced apoptosis in mega-dosed rats. Only the most alarmist commentators anticipate a delayed epidemic of demented depressives as a result of serotonergic carnage caused by MDMA abuse. But equally, no alien anthropologist in his right mind who merely read the gruesome scientific literature on MDMA would want to self-experiment with such a deadly neurotoxin. Taking weed-killer, glue sniffing or swallowing rat poison sounds marginally less dangerous. Calling it dystopian pharmacology might seem more apposite. Even listening to glowing, first-person accounts of the MDMA experience is curiously uninspiring when refracted through the lens of our normal Darwinian consciousness. The prospect of love, peace and empathy seems less exciting than a round of Quake 3. We are all prone to mood-congruent thoughts.
In any case, MDMA users themselves may find the magic of the initial drug-induced epiphany tends to fade with frequent use. For many but not all users, a magical drug becomes just a feel-good drug. Adverse side-effects tend to become more troublesome. Higher doses are needed to gain the same effect. Users lament that "the E isn't as pure as it used to be"; and that the tablets are weaker. Often indeed this is true; but a physiological explanation for so-called "cumulative tolerance" must be sought as well. Enzyme-induction plays a role, though the phenomenon isn't fully understood. Pharmacodynamic tolerance to a drug is normally reversible, yet some users of MDMA report they never quite recapture the initial ecstatic glory even if they abstain for a year or more. Researchers are still unsure if this fade-off is a symptom of long-term neuroadaptation or serotonergic damage.
Perhaps we shouldn't be so surprised at the "loss of magic". The liver (and the brain) is adapted to life on the African savannah. Our vital organs can't know the difference between the elixir of life and a poison. MDMA has the attributes of both, and in the African bush, the latter is a more realistic outcome. Yet we won't be trapped in brutish states of consciousness for ever. In the near future, functional analogues of MDMA promise to enhance mental health, add perpetual magic to our lives, and beautify our troubled minds. Empathetic bliss isn't inherently toxic; though its reactive metabolites may be. In principle, the psychopathologies of everyday life can all be cured. MDMA offers a foretaste of life in post-Darwinian paradise; but it delivers, at best, only a fleeting hint of the magic to come.
MDMA: neuroprotection
No safe, indefinitely sustainable entactogens-empathogens yet exist. Drugs that consistently induce the opposite syndrome are legion. Some such drugs are billion-dollar moneyspinners for Big Pharma. They are clinically licensed and widely prescribed in the guise of psychiatric medicines. Other psychoactive drugs are used mainly for "unrecognised" and non-medical purposes. Psychostimulants like cocaine and amphetamine notoriously promote egotism and aggression. Drinking ethyl alcohol tends to make the user relaxed, disinhibited and stupid.So is MDMA itself best reserved as a sacrament for special occasions? Or can it be safely taken "recreationally" and socially? What dosage, if any, is prudent? Is the MDMA experience so tantalising that it's best avoided altogether lest the rest of one's life pall in contrast? Would one want one's sixteen year-old daughter to take it; and with whom?
Currently the risk-benefit analysis of taking - or missing out on - MDMA is unclear. Probably the gravest threat to the long-term emotional and physical health of the user is getting caught up in the criminal justice system. Victims of the law-enforcement agencies frequently suffer long-term neuropathological changes. Lowered serotonin levels, elevated cortisol, confusion, depression, sleep problems, severe anxiety, and paranoia are common. In some cases, the neurological damage may be permanent. Currently around 500,000 "drug-offenders" languish in American jails alone; and millions more young people throughout the world are at risk. Yet repealing ill-conceived drug laws is only part of the answer in protecting mental health.
Ever more alarming animal studies conducted over a decade by George Ricaurte, a neurotoxicologist at John Hopkins University School of Medicine, suggest that taking high and/or frequent doses of MDMA causes damage to the terminals of serotonin axons in the brain. Cerebrospinal fluid 5-hydroxyindoleacetic acid (5-HIAA), serotonin's major metabolite which serves as a marker of central serotonin (5-hydroxytryptamine, 5-HT) neural function, may be lower in human MDMA users than in putatively matched controls. The number of serotonin transporter sites, structural protein elements on the presynaptic outer axonal membrane that recycle the released neurotransmitter, may be reduced too. Long-term MDMA-induced changes in the availability of the serotonin transporter may be reversible; but it is unclear whether recovery is complete. Currently the balance of neurochemical and neuroanatomical evidence, and functional measures of serotonin neurons, suggests that it is imprudent to take MDMA or other ring-substituted methamphetamine derivatives without also taking neuroprotective precautions. Arguably, it is best to take MDMA infrequently and reverently or not at all - Dr Shulgin once suggested a maximum of four times a year.
MDMA's apologists aren't convinced that the neurotoxicity evidence is persuasive - except for MDMA taken at unrealistically high doses. As Paracelsus (1493-1541) noted centuries ago, "All substances are poisons: there is none which is not a poison. The right dose differentiates a poison and a remedy." Most early studies of the possible long-term adverse effects of MDMA use in humans have been methodologically flawed - inadequately controlled, retrospective rather than prospective, and marred by a failure adequately to exclude confounding variables - e.g. the so-called stereotype threat. Some published toxicity studies include a large percentage of self-reported "Ecstasy" users who've never even taken MDMA. Other studies rely on a small minority of users whose drug-taking methodology owes more to Hunter S. Thompson than Sasha Shulgin.
Yet the biggest problem in evaluating the published evidence isn't so much sloppy science or value-judgements masquerading as statements of fact. It's rather that just as the strongest predictive factor in the outcome of a published clinical trial of any psychiatric drug is the identity of the funding body, likewise the investigation of MDMA isn't a disinterested search for scientific truth. Published papers that examine possible confounding variables in MDMA "toxicity studies" omit to mention the greatest biasing factor of all. Independent funding is critical to the integrity of biomedical research; but MDMA is now a Schedule One drug. Studies of MDMA can be lawfully conducted only under government license by ideologically-vetted researchers. Authors and licensed researchers are implicitly paid to show how prohibited drugs are harmful, not that they can be potentially therapeutic. Researchers certainly aren't paid to report that some illegal drugs are potentially life-enhancing agents. Nor do their paymasters expect them to investigate the design of safer, more sustainable analogues to improve the user experience.
Intuitively, at least, it might seem axiomatic that in a democratic free society every person should have "the license to explore the nature of his own soul" (Dr Shulgin). Yet this license has lately been revoked in the name of the War Against Drugs. Every law-abiding citizen is now locked into traditional modes of consciousness on pain of criminal prosecution and imprisonment. The chemical keys to the locks themselves have been outlawed. Most natural scientists are scornful of social constructivists who think that power structures underwrite the way we see the world. But in a daring extension of the Papacy's Index Librorum Prohibitorum, knowledge of entire state-spaces of potential experience has been outlawed following passage of the USA's Controlled Substance Analogue Enforcement Act of 1986. The UN's World Health Organization and foreign governments have been leaned on, bribed or dragooned into the War On Drugs too. In the USA itself, the world's most celebrated psychedelic chemist and leading authority on MDMA has been stymied from conducting human research on Schedule One compounds after publishing his trailblazing autobiography-cum-cookery book. Worried that his life's work might be quite literally destroyed by the drug-warriors, Dr Shulgin acted to thwart the obscurantists before it was too late. "I can see having maybe two or three people in the higher echelons of the government who may not like what I do, and I did not want particularly to have all of this be seizable and burnable, So I published it. Now you cannot get rid of it." Dr Shulgin had a DEA analytical license - a "Faustian bargain" according to MAPS's Rick Doblin. But in 1994, Dr Shulgin fell victim to a DEA raid on his research lab. Under the transparent pretext of "health-and-safety" infractions, Dr Shulgin's license to work with scheduled drugs was withdrawn.
Suppression of "illicit" knowledge in academia and the overground research community isn't normally so melodramatic or heavy-handed. But systemic bias and the habit of internalised self-censorship extends throughout the apparatus of peer-reviewed journals, sponsored conferences, and mainstream clinical medicine. On the one hand, negative results and non-results from toxicity studies are difficult to publish or publicise. Conversely, "positive" toxicity results from studies run by primate vivisectionists using chronic or near-fatal MDMA doses are newsworthy and fundable. Such publication bias is insidious and endemic; it's underestimated because prospective authors are broadly aware of what can - and can't - get published; and so they don't bother to submit what they know can't be accepted. Even this biasing factor massively understates the problem. This is because most potential psychedelic research projects can't get official permission or funding in the first place. As noted by New Scientist in Ecstasy on the Brain (April 2002): "'It's an open secret that some teams have failed to find deficits in ecstasy users and had trouble publishing the findings...The journals are very conservative,' says [Andrew] Parrott. 'It's a source of bias.' Parrott himself has had two papers of this sort turned down."
Of course bias cuts both ways. MDMA enthusiasts find it hard to write even-handedly too. Among MDMA's "unlicensed" and independent researchers, there is a natural tendency to believe any agent that triggers such sublime states must essentially be good for you. MDMA can indeed be life-transforming; but unless it's used sparingly and at conservative doses, it is still a potentially toxic drug. MDMA's defenders would say that the same is true of lithium, penicillin or paracetamol, none of which are banned.
Some studies suggest that possible MDMA-induced neurotoxicity to the serotonin system can be largely prevented by taking a double dose of fluoxetine (Prozac) or another SSRI shortly after starting to "come down". Post-E Prozac in particular mitigates the oxidative stress and consequent risk of serotonergic axon damage caused by reactive products of dopamine deamination. The long-acting SSRI Prozac/fluoxetine, and its even longer-acting metabolite norfluoxetine, apparently prevents the uptake of dopamine (and any toxic metabolite(s)?) into the serotonergic nerve terminals by binding to the serotonin reuptake transporter with higher affinity than MDMA or serotonin. Unfortunately, although liquid refreshment is now freely available at most MDMA-propelled raves, most chill-out rooms don't offer Prozac. Two days and more after taking MDMA, heavy recreational users are typically more irritable, subdued, unsociable and subtly less empathetic than before their weekend binge: the "Terrible Tuesday's" syndrome of midweek blues. So with cruel irony, two or three days after communing on Ecstasy and declaring their undying love, couples are more likely to have rows and split up. Other heavy regular MDMA users, even those who aren't self-medicating for a pre-existing malaise, may experience depression, anxiety, emotional burnout, rejection-sensitivity, fatigue, insomnia, aching limbs, subtle cognitive deficits, immune system dysfunction, body temperature dysregulation, and a sense of derealisation or depersonalisation for several weeks or months afterwards. This litany of woe sounds a high price to pay even for the peak experience of a lifetime.
Alas, adopting a prophylactic SSRI regimen isn't a realistic long-term option for frequent MDMA users either, or at least not if they intend to continue using their hugdrug of choice. This is because a sustained regimen of SSRIs largely blunts MDMA's empathogenic and entactogenic effects. SSRIs inhibit the binding of MDMA to the serotonin transporter. Thus pre-treatment with SSRIs prevents MDMA-triggered serotonin-release; and this in turn reduces dopamine-release in the striatum. Some SSRI users who like to rave nonetheless continue to take MDMA. They consume abnormally high quantities of pills to gain the desired E-like effect. At this dosage range, the persistence of metabolite-induced MDA-like states of consciousness the next day is not unexpected. In practice, the after-effects are often modulated by cannabis and alcohol.
Tolerance to MDMA itself develops quite rapidly with steady use. If MDMA is taken several days in a row, amphetamine-like and eventually dysphoric effects start to predominate. Monoamine neurotransmitters, most drastically serotonin, are depleted from the axon terminals; serotonin synthesis is choked off following oxidative inactivation of tryptophan hydroxylase; and the nerve-cell receptors re-regulate. Thus MDMA is not addictive in the conventional sense. Taken chronically, it soon ceases to be rewarding. Even dedicated ravers typically don't binge more than once a week. Wiser heads save the drug for "special occasions". Yet MDMA's non-addictive profile is no guarantee that (as was once fondly hoped), "once you get the message you hang up the phone." The mind/brain isn't built like that. If you really like a drug-delivered message, you want to hear it again and again. But with MDMA, the message can subtly change with time; and its primal magic gets sullied or forgotten.
There are other options for neuroprotection besides taking post-Ecstasy Prozac. On one hypothesis of MDMA-induced serotonergic neurotoxicity, the extra dopamine released into the synapses is transported into the depleted serotonin axonal terminals where it is deaminated by the enzyme monoamine oxidase type-B present in the terminal. MAO has two isoforms, MAO-A and MAO-B. These differ in their substrate affinities and inhibitor sensitivities: the MAO-A isoenzyme has a greater affinity than the MAO-B isoenzyme for serotonin, but mainly MAO-B is present in the serotonergic axonal terminals, where it breaks down "foreign" neurotransmitters. However, after a subject has taken a high dose of MDMA, excess dopamine is taken up by the so-called serotonin transporters into the depleted serotonin terminals. Here its oxidation produces a glut of toxic free radicals - highly reactive chemicals with one or more unpaired electrons - such as hydrogen peroxide (H2O2). These toxic free radicals are liable to exhaust or overwhelm the free radical scavenging systems of the cell. In consequence, the serotonin fine axonal terminals are broken down by lipid peroxidation. Why exactly the serotonin reuptake transporters lose their normal selectivity for serotonin and take up dopamine isn't known for certain. Possibly it's because by this time there's far less serotonin around for the reuptake pump to use. After the directionality of the reuptake pump is reversed by MDMA, serotonin released into the synapse can't be recycled back into the cell; and so it diffuses away. In any event, the monoamine oxidase inhibitor selegiline [l-deprenyl/Eldepryl] appears to be neuroprotective at monoamine oxidase type-B-selective dosages i.e. 2 x 5mg daily or less. Selegiline also protects against MDMA-induced inhibition of tryptophan hydroxylase. Interestingly, Prozac too has MAO-B inhibiting properties; and these may contribute to its neuroprotective effect. Selegiline itself has additional free radical scavenging properties that may exert a neuroprotective action. It will be instructive to compare the neuroprotective efficacy of selegiline with rasagiline (Azilect, Agilect) for E-users. Rasagiline is a selective MAO-B inhibitor licensed from mid-2005 in the EC for the treatment of Parkinson's disease; rasagiline lacks selegiline's trace amphetamine metabolic by-products. Whatever the older compound's neuroprotective efficacy compared to rasagiline, selegiline is potentially valuable too because, unlike taking a SSRI, adopting a long-term selegiline regimen doesn't impair MDMA's subjective effects. Even so, no controlled clinical trials of their co-administration are currently planned.
One reason for such caution beyond a reflex Just-Say-No dogmatism is that it's potentially dangerous to tamper with the MAO enzyme. Selegiline has lifespan-extending properties in "animal models", and possibly in humans too; but if used recklessly, then it could abruptly shorten life instead: selegiline is an irreversible MAO-B inhibitor. Prohibitionism and a consequent absence of quality-control means that the "Ecstasy" sold in clubs often contains liberal quantities of amphetamine. Amphetamine and MAO inhibitors should not be combined. Both enantiomers of MDMA itself have MAO-inhibiting effects, preferentially for isoenzyme type-A. Taken at dosages of above 2 x 5mg per day, selegiline loses its selectivity for MAO-B. Individual variation in MAO status makes it imprudent for the MDMA user to take selegiline even at 10mg daily; and selegiline itself, like MDMA, is a weak inhibitor of MAO-A. MAO-A deaminates serotonin; and the serotonin syndrome, characterised inter alia by hyperthermia, autonomic instability and altered muscle tone, is potentially lethal. Serotonin 5-HT2A antagonists like ketanserin (Sulfrexal) can inhibit the syndrome; but they aren't widely available on the street or average dance-floor.
Milder cases of the serotonin syndrome are not uncommon among the hard-rolling stackers and piggybackers dancing all night at crowded ill-ventilated raves. Dehydration and overcrowding tend to worsen drug-induced toxicity. Heat exhaustion and severe hyperthermia are probably the gravest risk to the raver's health. MDMA tends to raise body temperature by a degree or so, sometimes by quite a bit more if the user dances all night without rest ["Saturday night fever"]. MDMA also increases the body's secretion of antidiuretic hormone, arginine-vasopressin. Ravers sometimes overcompensate for the risk of dehydration by gulping down too much pure water. This can cause hyponatraemia (literally "low salt": "water intoxication"). Sipping a couple of sports-drinks every hour or so instead is a prudent way to maintain electrolyte balance. Indeed it would be safer if sports drinks were distributed with each E-tablet sold as a matter of course, perhaps accompanied with a neuroprotectant mix and a health-tips sheet thrown in for good measure.
Unfortunately, tips found on the Net are no substitute for systematic, well-planned health-education programs. Organisations like the Berkeley-based Dancesafe, funded by Microsoft millionaire the late Bob Wallace and founded to promote safe raving, are rare; their activities are also controversial. Until psychopharmacology becomes part of the educational core curriculum, any responsibly designed drug cocktail, and any harm-reduction program, must be formulated with the recklessness of a minority of sensation-seekers in mind, not just risk-averse research scientists. Such a revolution in mental healthcare for young people is sorely needed. An examination system akin to ritualised child-abuse wreaks terrible damage on the young minds incarcerated in our educational institutions. Critics of exam-culture claim an "education" based around competitive testing screws kids up far more than empathetic drugs. Unfortunately, what's tested in these rituals of abuse isn't our children's emotional well-being, levels of reflective self-insight, capacity for loving empathy or social intelligence. Nor do schools and colleges offer courses in effective technologies to promote them.
A healthcare revolution of this magnitude isn't going to happen tomorrow. So more realistically for now, clubbers seeking neuroprotection against MDMA-induced toxicity may do well to use humble antioxidants such as ascorbic acid (Vitamin C), alpha-tocopheryl-acetate (Vitamin E), zinc, alpha-lipoic acid, and L-cysteine. The optimal mix and dosage before, during, and after dropping an E to maximise their respective neuroprotective action, and minimise any post-ecstatic hangover, hasn't yet been established. Even at high dosage, the neuroprotection such antioxidants offer may be inadequate for heavy MDMA users. More encouragingly, antioxidants also reduce tolerance between exposures. Clearly a lot more research is needed, hopefully without the usual animal holocaust that accompanies drug testing today.
The serotonin precursors L-tryptophan and 5-hydroxytryptophan (5-HTP) are also neuroprotective against MDMA-induced toxicity, possibly in part because of their antioxidant effect but mainly because of their precursor role. 5-HTP is the metabolite of L-tryptophan. It's the direct metabolic precursor to serotonin (5-HT). In contrast to the catecholamine neurotransmitters dopamine and noradrenaline, the synthesis of serotonin isn't subject to strong end-product inhibition. Like L-tryptophan, 5-HTP is sometimes used as an antidepressant and antianxiety agent; it seems to have a relatively narrow therapeutic window. Unlike SSRIs, L-tryptophan and 5-HTP can be taken chronically without blunting MDMA's effects. Indeed some clubbers pre-load with L-tryptophan or 5-HTP to intensify and enrich the MDMA experience and prevent serotonin depletion. Serotonin depletion increases the vulnerability of the axon terminals to damage. Though such a tactic is sensible enough in theory, excess preloading with 5-HTP may potentially precipitate or exacerbate the serotonin syndrome. So care is in order.
With or without 5-HTP supplementation, an idealised stone-age diet can be especially valuable for heavy MDMA users. Contrary to a once widely-propagated but now discredited myth, Merck never planned to develop MDMA as an appetite-suppressant. Yet the company might well have done so: MDMA's appetite-suppressing effect is quite strong. Drugs that directly or indirectly activate the serotonin 5-HT1B and 5-HT2 receptors tend to be anorexiants. Lazy and reluctant eaters who regularly take Ecstasy are at greater risk of vitamin and mineral deficiencies, and more vulnerable to MDMA-induced serotonergic damage, than matched controls.
One novel and unlikely-sounding proposal to minimise MDMA-induced neurotoxicity is pretreatment with aspirin. Aspirin inhibits the enzyme prostaglandin H synthase (PHS). PHS catalyses the transformation of amphetamines into toxic free radical products. Therefore taking aspirin before MDMA use may also indirectly block the conversion of amphetamines into reactive oxygen species responsible for long-term neurotoxicity. As of 2014, no controlled trials of aspirin have yet been conducted with MDMA-using humans. But if aspirin pretreatment does prove an effective harm-reduction strategy, then this is potentially a godsend - not least because other candidate neuroprotectants (SSRIs, selegiline, etc) carry hazards of their own in conjunction with E-use. Aspirin itself cannot strictly be described as risk-free; but the risk/benefit ratio of its use is both favourable and well-known.
However, the biggest long-term obstacles to preventing neurotoxicity and drug-related mental health problems are ideological, not pharmacological. The discovery that MDMA is not always the harmless fun-drug that a number of its recreational users (understandably) first supposed has caused the medical establishment to demonise MDMA or dismiss its psychotherapeutic potential completely. Critics of the drug-warrior mentality claim that MDMA's possible neurotoxicity served only as a pretext for banning it. The case for making, say, tobacco a schedule-one drug isn't notably less compelling, nor would any rush to judgement on the safety, medical use or addictive potential of tobacco-products seem so premature. The size of the cumulative death toll in the tobacco epidemic almost defies comprehension: yet we continue energetically to market a lethal drug to hundreds of millions of youngsters in the Third World. The contrast between the treatment of dealers in tobacco products and MDMA distributors couldn't be much starker. Instead of aiming to prevent possible MDMA-induced neurotoxicity by tweaking or enhancing the agent in question, or designing better functional analogues, or seeking ways to antagonise possible toxic metabolites, or running health campaigns promoting the co-administration of free radical scavengers or other neuroprotectants, the authorities opted simply to outlaw MDMA altogether. Users and independent researchers alike were criminalised. Scientific investigation was crippled. MDMA was driven underground, where it could mix with innumerable contaminants and organised crime.
Fortunately, scientific research on MDMA has lately revived, albeit under license and mainly on non-humans. Rats and monkeys are in some ways uncannily similar - genetically, behaviourally and biochemically - to human beings. Both the electrical-signalling properties and molecular machinery of neurons are widely conserved across the animal kingdom. There are interspecies differences e.g. MDMA is anxiogenic rather than anxiolytic in some mouse strains at low doses; MDMA administered to rats in cold ambient temperatures induces hypothermia; and MDMA causes opposing sensorimotor gating effects in rats and humans. Yet the fundamental similarity of "animal models" to human beings is precisely why we use, vivisect and then "sacrifice" our fellow creatures in drug discrimination studies and medical research. Using principles of interspecies scaling, it is possible to estimate the crude physical effects of comparable MDMA doses on people after conducting animal experiments, although species differences in MDMA's pharmacokinetics and active metabolites make the details of such scaling controversial. If oxidative metabolites, not MDMA itself, are responsible for neurotoxicity, then investigation of the particular ways MDMA is metabolised in humans will be critical in determining safe dosages. But beyond the narrow physical effects of MDMA on the brain, it's hard enough for articulate humans who take insight-and-empathy drugs to verbalise their processes of introspection. What can we learn about entactogenesis by mega-dosing a rhesus monkey? All sorts of intellectually fascinating physiological data can be gleaned from experimenting on live animals - and even more data from experiments on live humans. Yet ethically, how can we humanely experiment on members of other species when we can't "predict whether a particular molecule will open the gates of heaven or stoke up the fires of hell" (Dr David Nichols). Clearly non-humans can't describe the effects on their consciousness of psychoactives, even though they can be taught to "discriminate" them - the so-called "animal model" of subjective drug effects. So members of other species can't describe the illegal knowledge drug-naïve humans are missing out on - or the horrors we inflict on their minds and bodies.
Even if animal research throws up a true wonderdrug ideal for human use - a safe, sustainable miracle-pill with a well-defined therapeutic window, life-enriching subjective profile, and no significant adverse side-effects - then under today's regulatory regime, the potential wonderdrug couldn't get a product-license. In law, only medicines to treat well-defined clinical disorders can be licensed, not investigational agents designed to enhance our quality of life or enrich "normal" human mental (ill-)health. Short of labelling the agent as a "food supplement" - which might be stretching it a bit for MDMA and its analogues - true pharmacological life-enrichers will be condemned to legal limbo. Even if this perverse restriction on legal drug availability were lifted, then any prospective blockbuster most likely still wouldn't get regulatory approval in practice. Human clinical trials cost tens of millions of dollars to run. MDMA itself has long been off-patent. So profit-driven pharmaceutical companies aren't interested in funding pilot studies. Empathy-And-Insight Deficiency-Disorder isn't covered in DSM-IV, the psychiatrists' bible. A condition that isn't medically acknowledged can't be treated by state-licensed pharmacotherapy.
The gloom-and-doom shouldn't be overdone. Eventually, safe long-lasting E-like super-cocktails and enhanced functional analogues of MDMA may indeed be both patentable and judged therapeutic for "officially" sanctioned medical conditions such as anti-social personality disorder, refractory depression, PTSD, Asperger syndrome and autism. These super-cocktails and sustainable MDMA analogues should prove life-enhancing for "normal" self-regarding people who would like to improve themselves too. Such usage may or may not stay "off-label"; but it needn't be illegal.
In the meantime, bitter experience of the hedonic treadmill of Darwinian life instils a reluctance to believe anything so magical as the MDMA experience could be sustained and enriched indefinitely. ["You can't have the sweet without the sour"; "You need pain to appreciate pleasure", etc.] But such superstition is pre-scientific; it may soon seem quaint. As intracranial self-stimulation studies attest, pure pleasure induced by electrical stimulation of the ancient mesolimbic pleasure centres of the brain shows no tolerance. Response- and remission-rates are 100%. In the present era, depression, self-ignorance and sociopathy are demonstrably sustainable over a lifetime; but so, in theory, are the biochemical substrates of happiness, lucid self-insight and even saintly empathetic bliss. The hedonic treadmill can be dismantled, its inhibitory feedback mechanisms redesigned, and its neurogenetics rewritten. In principle, and perhaps one day in practice, we can be nicer, happier and smarter indefinitely. Unfortunately this utopian outcome won't result from a chronic regimen of MDMA.
Ecstasy for life?
What are the presently available options for enhancing and extending the MDMA experience? Two separate questions need to be distinguished. First, what if any drug or drug-cocktail can safely replicate the acute subjective effects of MDMA? Second, what if any drug or drug-cocktail or gene-therapy can best induce E-like consciousness over the long-term?The holy grail of safe, sustainable entactogen-empathogens almost certainly won't be found in the guise of structurally-tweaked chemical homologues of MDMA. A long-term regimen doesn't seem feasible even if the exact structural requirements needed to reproduce MDMA's acute stimulus effects were understood. To make the magic last for ever, or at least to induce it at will over several decades, only a long-lasting homeostatic re-regulation of the central nervous system will work. Thus the substrates of a lifelong capacity for E-like consciousness can't be engineered via, say, a mechanism akin to the MDMA-induced reversal of the serotonin reuptake pump. Depleting the brain's serotonin or, even worse, inhibiting the molecular machinery needed for its renewed synthesis, is a recipe for clinical depression, not Heaven-on-Earth. Nor can the substrates of perpetual empathetic bliss be delivered by tonic stimulation of the same pre- and post-synaptic receptors activated by an acute flood of extra serotonin/dopamine in the synapses - or at least not in the same way. Prolonged receptor activation typically leads to receptor desensitisation and/or down-regulation. The inhibitory feedback mechanisms that keep our Darwinian brains so mean-spirited need to be sabotaged, not kicked into gear.
Such a profound homeostatic shift in normal waking consciousness might conceivably be delivered by functional analogues of MDMA. The idea here would be to reproduce E-like euphoric and empathogenic-entactogenic effects, not acutely, but via delayed receptor subtype-specific re-regulation. MDMA itself rapidly depletes serotonin from the axon terminals and inactivates the enzyme tryptophan hydroxylase needed for its renewed biosynthesis. By contrast, altering the density and signal-transduction efficiencies of the mission-critical receptor subtypes [5-HT1B(?), 5-HT2A(?), dopamine D2(?)], could, ideally, deliver sustained ecstasy without emotional burnout. Such receptor re-regulation might involve a time-lag of one-to-three weeks, as is normal with conventional "antidepressants". Delayed-onset magic, if achievable, would offer an immense social and therapeutic advantage. This is not just because the magic should be sustainable without limit, but because postponing the onset of drug-induced reward minimises a medicine's "abuse-potential" without compromising its efficacy. The practice of tobacco-smoking, for instance, is so addictive not because of the surpassing joys of inhaling a cigarette, but because a tobacco abuser need wait only seven seconds or so between taking a puff and the miniscule hit. The reward from oral MDMA takes somewhat longer; but the delight of E-like consciousness needs to be divorced from its intimate association with pill-popping.
Alas the brain's post-synaptic signal-transduction mechanisms aren't yet sufficiently understood to bring about a magical E-like re-regulation of waking consciousness indefinitely. Inducing lifelong egoistic bliss is less of a technical challenge. Wireheading is uniquely effective, though most of us might prefer the services of a molecular psychiatrist to a neurosurgeon. Fortunately, our options may soon extend beyond the crudely hedonistic. Indeed within a few decades, taking a controlled-release maintenance dose of functional analogues of MDMA may seem as natural as swallowing a multivitamin pill; and just what the doctor ordered.
Alternatively, the neurochemical substrates of MDMA-like magic may be preserved, or continually re-created as desired, via a cocktail of agents rather than monotherapy. On this approach, each individually designed ligand would be targeted selectively at the potentially magic-signalling receptor subtypes [or at second-messenger pathways coupled to the G-protein-linked signal-transduction system, or in theory direct mechanisms of gene regulation and expression]. This may be feasible; but its orchestration will be much harder than it sounds. As the catalogue of serotonin receptor subtypes has grown, so has our library of serotonergic molecular probes; yet so too has a realisation that agents previously reckoned to be selective for a particular class of serotonin receptor are less selective than originally claimed. Hence there is a need for novel agonists, antagonists and inverse agonists with far greater selectivity for each receptor subpopulation. This multiple targeting strategy is technically challenging, but it's probably more promising than relying on a single "dirty" non-specific indirect serotonin agonist like MDMA. Although there are indeed other, non-neurotoxic amphetamine derivatives that acutely induce transporter-mediated serotonin release, achieving the all-important goal of sustainability may entail the use of drug cocktails. Thus one might explore combining e.g. 1] new synthetic allosteric modulators of the serotonin 5-HT1B autoreceptors that regulate the evoked release and synthesis of serotonin; 2] agents acting selectively on the 5-HT1B-autoreceptors and heteroreceptors; 3] the right 5-HT2C receptor antagonist or inverse agonist to make the E-like state more ecstatic; 4] the right dopaminergic(s) or, ideally, agents targeting the medium spiny GABAergic projection neurons in the rostromedial shell of the nucleus accumbens directly. This is all still very speculative and unfunded.
Alternatively, and perhaps more plausibly, locking in the neural substrates of empathetic bliss as a default-state of consciousness may be achievable only via gene therapy, or perhaps a hybrid gene-and-drug combination treatment. One option here would be inserting "good" variants of the tryptophan hydroxylase gene, which codes for the rate-limiting enzyme of serotonin biosynthesis, and then once again co-administering receptor subtype-selective ligands and/or serotonin releasers. Our immediate options are limited. Pharmaceutical interventions aimed at extending, for example, profound emotional depth over many decades rather than a few hours may entail, not flooding the synapses with extra serotonin followed by extra dopamine release as in acute dosing with MDMA, but instead using e.g. serotonin reuptake accelerators analogous to the memory-enhancing antidepressant tianeptine (Stablon); or perhaps enhancing the love-and-nurturance-promoting oxytocin system; or perhaps using better designed analogues of the emotion-deepening agent Gamma-HydroxyButyrate (GHB).
A brief comparison of GHB and MDMA may be instructive because one therapeutic challenge ahead will be to design agents that reverse SSRI-like flattening of affect without inducing mawkish sentimentalism (cf. ethyl alcohol). In contrast to mainstream psychiatric drug therapies, both GHB and MDMA deliver a rare emotional intensity of experience, albeit an intensity different both in texture and molecular mechanism. GHB is known by clubbers if not structural chemists as "liquid ecstasy". GHB and MDMA are indeed sometimes mixed at raves; but the two drugs are chemically unrelated. GHB is an endogenous neuromodulator derived from GABA, the main inhibitory neurotransmitter of the brain. A naturally-occurring fatty acid derivative, GHB is a metabolite of normal human metabolism. GHB has its own G protein-coupled presynaptic receptor in the brain. Sold as a medicine, GHB is licensed as an oral solution under the brand name Xyrem for the treatment of cataplexy associated with narcolepsy. Unlike MDMA, GHB stimulates tissue serotonin turnover. GHB increases both the transport of tryptophan to the brain and its uptake by serotonergic cells. Taking GHB stimulates growth hormone secretion; hence its popularity with bodybuilders. GHB offers cellular protection against cerebral hypoxia, and deep sleep without inducing a hangover. GHB also stimulates tyrosine hydroxylase. Tyrosine hydroxylase converts L-tyrosine to L-dopa, subsequently metabolised to dopamine. Unlike MDMA, the acute effects of GHB involve first inhibiting the dopamine system, followed the next day by a refreshing dopamine rebound. GHB induces mild euphoria in many users. In general, the neurotransmitter GABA acts to reduce the firing of the dopaminergic neurons in the tegmentum and substantia nigra. The sedative/hypnotic effect of GHB is mediated by its stimulation of GABA(B) receptors, though GHB also modulates the GABA(A) receptor complex too. The main effect of GABA(B) agonism is normally muscle relaxation, though interestingly, pretreatment with the GABA(B) agonist baclofen also prevents an MDMA-induced rise in core body temperature. Whatever the exact GABA(A), GABA(B), and GHB-specific mechanisms by which GHB works, when taken at optimal dosage GHB typically acts as a "sociabiliser". This is a term popularised by the late Claude Rifat (Claude de Contrecoeur), author of GHB: The First Authentic Antidepressant (1999). Rifat was GHB's most celebrated advocate and an outspoken critic of Anglo-American psychiatry. Similar therapeutic claims have been made for GHB as for MDMA, despite their pharmacological differences. GHB swiftly banishes depression and replaces low mood with an exhilarating feeling of joy; GHB has anxiolytic properties; it's useful against panic attacks; it suppresses suicidal ideation; it inhibits hostility, paranoia and aggression; it enhances the recall of long-forgotten memories and dreams; and it promotes enhanced feelings of love. Like MDMA, and on slightly firmer grounds, GHB has been touted as an aphrodisiac: GHB heightens and prolongs the experience of orgasm. GHB disinhibits the user, and deeply relaxes his or her body. Inevitably, GHB has been demonised as a date-rape drug ["I was at this party, and this guy gave me a drink. Next thing I know, it's morning and I'm in someone's bed. I've no idea what happened in between..."]. GHB has a steep dose-response curve. Higher doses will cause anterograde amnesia i.e. users forget what they did under the influence of the drug. It's dangerous to combine GHB with other depressants. So despite GHB's therapeutic and pro-social potential, GHB is probably unsafe to commend to clubbers. This is because a significant percentage of the population will combine any drug whatsoever with alcohol regardless of the consequences to health. If used wisely, sparingly, and in a different cultural milieu, then GHB could be a valuable addition to the bathroom pharmacopoeia. But even then, it's still flawed. GHB may intensify emotion and affection, but not introspective depth or intellectual acuity. Unlike taking too much MDMA, overdoing GHB makes the user fall profoundly asleep. If our consciousness is to be durably enhanced, then sedative-hypnotics have only a limited role to play in the transition ahead.
So what are the prospects for richer, intenser, sustainable insight-and-empathy drugs from MDMA's phenethylamine sisters and cousins?
Post-Shulgin, the quantified structure-activity relationships of MDMA and related compounds (MDEA, MDA, MBDB, MMDA,, etc) have been investigated, even though systematic overground exploration of their effects on the human psyche has been strangled at birth. Research chemists have designed a host of ring-substituted amphetamine derivatives with one or more substituents attached at different positions to the phenyl ring of the amphetamine or methamphetamine structure. Other such derivatives have been devised by entrepreneurs whose synthesis of designer drugs aims more at circumventing legal restrictions than pushing back the frontiers of knowledge. In general, different monoamine-releasing amphetamine analogues become less subjectively rewarding as their serotonin-releasing potency is increased relative to dopamine-releasing potency.
Whatever their parentage, the phenethylamines as a whole exert a spectrum of action from the purely stimulant activity shown by "noradrenergic/dopaminergic" amphetamine to the almost entirely psychedelic activity of the "serotonergic" DOM - distributed at ultra-high doses in Haight-Ashbury San Francisco 1967 under the name of 'STP': Serenity, Tranquillity, and Peace. In drug discrimination studies, MDMA's subjective effects only partially cross-generalise to DOM and amphetamine. Indeed MDMA only partially cross-generalises to the other two hypothetical family prototypes currently identified, PMMA [N-methyl-1-(4-methoxyphenyl)-2-aminopropane] and TDIQ [5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline] from the "fourth dimension". MDMA itself is truly "one of kind" (Dr Shulgin), both structurally (i.e. the effects of the N-methylation of its primary amine, exclusive 3-4 di-substitution on the aromatic ring, its anomalously potent (+)-enantiomer) and subjectively. There's no obvious new tweak of its molecular structure, or to structurally related agents, that promises to deliver SuperEcstasy Mark 2, or even if there were, to suppose the supermagic would be truly sustainable. Thus MDMA's immediate homologue and closest relative, MDEA (3,4-methylenedioxyethylamphetamine: "Eve"), formed by swapping the 1 carbon methyl group for a 2 carbon ethyl group, is an interesting agent in its own right, but it's not going to deliver lifelong empathetic bliss. Indeed MDEA is actually less warm and empathetic, and more introverted in its typical subjective effects, than its sister molecule. Instead of taking MDEA as the racemate, one option is administering only (+)-MDEA, the optical isomer responsible for racemic MDEA's entactogenic quality. Yet pure preparations of individual enantiomers are not always readily to hand, nor a route to lifelong wisdom if they were.
Curiously, the beta-ketone analogue of MDMA, methylone (3,4-methylenedioxymethcathinone, MDMCAT), is poorly researched. Only a handful of papers appear in the published scientific literature. Methylone is another creation of Dr Shulgin. The drug is sold (expensively) as a "research chemical" over the Net. Branded rather unsubtly as "Explosion", methylone is also available in several Dutch smartshops. Recently it has become very popular in the scientific counterculture. The DEA issued an emergency ban on methylone on October 21, 2011. The drug is not as potent as MDMA; and it has a higher dosage range. Methylone causes less inhibition of serotonin reuptake and triggers less serotonin release than MDMA; but its potency in promoting the synaptic accumulation of the catecholamine neurotranmitters noradrenaline and dopamine is similar. Thus methylone has activating and empathetic effects while inducing less emotional outpouring. Many subjects experience an E-like "magic", though the two drugs can readily be distinguished by experienced users. It should be stressed that the comparative safety of methylone has not yet been well established, even at relatively low dosage levels of 120-150mg. This is still a new drug. Methylone is mood-elevating; higher doses induce a clear-minded and serene euphoria. Reputedly there is less serotonergic toxicity than MDMA; but there can still be a very noticeable comedown. If methylone is taken chronically, its stimulant effects become more pronounced. Its empathogenic qualities diminish. Tolerance soon sets in: a sad and familiar story. Likewise, the empathetic euphoriant mephedrone (4-Methylmethcathinone; 2-Methylamino-1-p-tolylpropan-1-one) can be acutely rewarding; but it is a short-acting stimulant whose pharmacokinetics and toxicology are unknown. Alas our knowledge (2014) of its properties comes wholly from user reports rather than peer-reviewed scientific journals.
From a theoretical perspective, PMMA [N-methyl-1-(4-methoxyphenyl)-2-aminopropane] a structural hybrid of paramethoxyamphetamine and methamphetamine, is interesting. PMMA arguably better represents a pure entactogenic [inward-looking, self-accepting, peaceful] family prototype than MDMA. However, the warm self-acceptance and empathetic love of others experienced on MDMA feels so clean and pure precisely because its mechanism is so messy. PMMA, on the other hand, lacks MDMA's residual psychedelic or speedy effects: PMMA is thus clearly distinct from the other hypothetical family prototypes, DOM or amphetamine, and also from TDIQ, about whose psychotropic effects rats currently know more than Homo sapiens. Unfortunately PMMA, like most methoxylated amphetamines, is potentially neurotoxic. In any case, it's completely unsustainable in regular use, though its ortho-isomer, methoxyphenamine, was once UK-licensed in tablet form as the bronchodilator Othoxine. PMMA itself is a potent drug with a very low therapeutic index: the combination of serotonin-release and MAO-A inhibition integral to its entactogenic profile makes it hazardous in overdose. In general, taking MAO-inhibiting agents with anything serotonergic is normally contraindicated because of the risk of the serotonin syndrome.
PMMA's reduced dopamine-releasing action makes it less "abusable" than other family members with overlapping psychostimulant effects. Yet rather than scorning the pleasure principle by seeking to minimise drug-induced reward, it might instead be more rational to design safer, benignly addictive lead compounds that maximise the user's well-being in lastingly empathetic, entactogenic and socially responsible ways. Well-designed (or serendipitously rediscovered) empathetic euphoriants can trigger socially responsible happiness. This is the distinctively E-like happiness that inspires love, nurturance and understanding rather than egotism and dominance behaviour. It's hard to imagine that any such futuristic love-drugs won't be "abusable" too. But if a drug isn't remotely rewarding or habit-forming, then it probably isn't any good. In the immortal words of Jeremy Bentham...
"Nature has placed mankind under the government of two sovereign masters, pain and pleasure...they govern us in all we do, in all we say, in all we think: every effort we can make to throw off our subjection, will serve but to demonstrate and confirm it."Alas application of means-ends rationality is rarely the norm in drug-policy debate or in psychiatric medicine. Nor is the pursuit of happiness undertaken much more rationally elsewhere. Thus we continue with Rube-Goldbergish efforts to improve our well-being via environmental scene-shifting - with mixed success.
Of course the biological route to nirvana has its share of pitfalls too; and MDMA is merely one of its most alluring seductions. Seekers of sustainable ecstasy would be rash to fetishise any particular drug or family of pharmacological tools - however magnificent their acute action on the user. For what matters, presumably, is the otherwise inaccessible modes of experience such agents can unlock in the mind/brain - and ways to sustain them - not the chemical structure of the agent that happens first to disclose their existence. "All science is either physics or stamp-collecting", Rutherford provocatively once proclaimed; and if some organic compounds didn't have the potential to unlock the doors to the kingdom of heaven, then Rutherford might have been right. As it is, school chemistry-lessons and standard textbooks rarely set young imaginations ablaze. They might conceivably do so if the PiHKAL-inspired compounds they ought to contain evoked the magical experiences their structures should ignite. Yet even the most astonishing centrally active compounds are only research tools or therapies, not sacraments. At least until we can genetically enrich the human mind/brain, no drug or research chemical, nor indeed any irritation of the body's surface sensory transducers by the environment, can do more than select from a pre-existing menu of brain-states composing the subject's mind/virtual world. In this sense, we're trapped.
Fortunately there is an escape-route; the false prison can be transcended. Within a few decades, the insertion of entirely new genes and variant alleles into our genome promises to revolutionise our stunted Darwinian minds. Novel neurally-expressed polypeptide sequences should disclose modes of experience hitherto unknown. The creation of genetically enriched neurons should allow the exploration of multidimensional search-spaces of consciousness which we presently lack the molecular wetware to imagine or even name. No psychoactive drug currently gives access to these hypothetical state-spaces. Such modes of consciousness have been barred to us by natural selection. They either diminished their user's Darwinian fitness or would have entailed crossing gaps in the evolutionary fitness landscape to get there. Whereas merely E-like states are normally inaccessible because their owners would get eaten or outbred, these unDarwinian modes of consciousness are quite possibly orthogonal to anything accessible today within our existing mental architecture. Each new state-space may be as different from the others as is sound from vision, or volition from cognition or emotion. The differences in gene-expression profile between neurons mediating the experience of, say, colour, or disgust, or humour (or being loved-up) may strike us as subtle. Yet the subjective differences in texture ("what it feels like") that their respective post-synaptic intracellular cascades generate are clearly spectacular. Who knows what else is accessible from Nature's psychoactive library by means of even "trivial" molecular genetic tweaks to our nerve cells? Disparate new categories of experience, and hopefully revolutionary conceptual schemes to navigate them, are presumably waiting to be unlocked just by inserting new sets of neurological instructions. Unfortunately we lack any God's-eye taxonomy of consciousness that might let us act like physicists and "carve Nature at the joints". The lack of an overall map, or even the ghost of a theory of consciousness to guide us, makes it impossible to place MDMA, or the spectrum of altered experience disclosed by psychedelic amphetamines, within any adequate scheme of classification. "Empathogen", "entactogen", "entheogen", and "psychedelic" are provisional and theoretically ill-motivated terms. A mature psychoactive taxonomy will need to be formulated relative to the architecture of particular phenotypes of mind, not the structure and pharmacology of the molecular probe alone. Alas the results of animal "drug discrimination studies" are no substitute for explanatory depth. In practice, today's psychonauts are reduced to describing the subjective effects of psychoactive drugs by contrasting them with their "normal" states of being. Inevitably this is all a bit lame. In retrospect, today's entire dreaming and waking consciousness may prove to be only minor variants on a theme whose motif can't be grasped from within.
Needless to say, the genetic choices, varieties of drug habit and modes of consciousness of our post-human descendants are a matter for conjecture. We've barely begun to ring the changes within the state-space of consciousness we've got. In order to replicate and sustain the family of MDMA-like magical states safely and reliably, it's necessary first to find the specific neurochemical signature of the family of enchanted states we're targeting. Thus by using, for example, transgenic receptor-knockout "animal models", SPECT (Single-Photon Computed Tomography), PET (Positron Emission Tomography) and MRI (Magnetic Resonance Imaging) scans, quantitative EEG with dense-mapping electrode arrays, antisense regulation of protein expression, and pre-treatment with other pharmacological ligands that activate or antagonise or act as inverse agonists at particular subtypes of receptor in the brain, it should be possible for ideologically committed bioscientists to discover what is crucial - and what's unwanted or inessential - to MDMA's psychological and physiological effects. Once the E-like signature is established, neuroscientists can then work how to mimic, refine and extend its magic, even if sustainable ecstasy is only a staging-post on the route to a richer biochemistry ahead.
First, however, MDMA's acute adverse side-effects i.e. teeth-grinding ["bruxism"], jaw-tension ["trismus"], loss of coordination ["ataxia"], eye-wiggling ["nystagmus"], profuse sweating ["diaphoresis"], nausea, appetite-suppression, tachycardia, dry mouth, hyperthermia or idiosyncratic reactions to MDMA need to be eliminated and not just minimised. The really nasty stuff - hepatotoxicity, cardiac arrhythmias, hyponatremia-induced cerebral and pulmonary edema (caused by drinking too much water), rhabdomyolysis (the breakdown of skeletal muscle), and disseminated intravascular coagulation (inappropriate blood-coagulation leading to severe bleeding) are statistically very rare. MDMA-induced incidence of these syndromes was apparently unknown in clinical practice prior to the drug's legal proscription. However, not all the problems of MDMA use can be blamed on Prohibition and the lethal mix of ignorance and criminality it spawns. Even pure, low-dose MDMA does not suit everybody. In the era of pre-genomic medicine, atypical reactions to any drug at all should be expected. Conversely, with adequate medical research the mildest bad experience on MDMA should be preventable.
Much more speculatively, the use of personalized somatic gene-therapy may enable future scientists of the mind, or unabashed hedonists, to sustain an otherwise neurotoxic drug regimen in safety. For instance, transgenic mice carrying the sequence of the human CuZn superoxide dismutase enzyme are resistant to MDMA-induced serotonergic damage. Ideology aside, humans can benefit from genetically enhanced neuroprotection no less than intoxicated rodents. If ever we wish to adopt a potentially life-enhancing but otherwise hazardous drug-regimen indefinitely, then one option may be to protect ourselves by inserting new genes or new alleles into our legacy genome. Or we may simply induce the overexpression of endogenous antioxidant enzymes already coded for. We're already on the brink of tailoring our drugs to our genes, but in principle we can tailor our genes to our drugs. Or we may choose to design, insert, and switch on and off as desired a suite of structural and regulatory genes for whatever life-enriching chemical exotica (or old favourites) we seek to enjoy. The modes of experience they generate may thereby become available, as it were, on tap. Nature uses lateral gene transfer; and rationally, so can we. Or by contrast, it's possible some or all genetically enriched post-humans may shun adulterants of their beautiful forms of consciousness altogether. If one's soul has been purified, why defile it?
To suppose that we might opt deliberately to micromanage even a subset of the thousands of neurally active genes of one's genome, and intervene to regulate their complex post-transcriptional editing, sounds far-fetched, even as the biotech revolution gathers pace. The prospect that we might personally choose to enrich our genetic repertoire from an ever-expanding library of newly-created DNA sequences, and an ever vaster neuroactive proteome, sounds still more remote. Could we really cope with such an enlarged freedom of choice? In practical terms, and perhaps surprisingly, yes. Sustainable E-like consciousness is just one option among myriad flavours of sentient existence. Radical enhancements of, say, the sorts of user-friendly visual interface we rely upon to interact with our PCs today can potentially be exploited to manage the neuroactive expression and regulation of one's individual genotype. End-user ignorance of the low-level molecular machinery is fully compatible with everyday expertise acquired in managing the kinds of consciousness one's genes code for - and mastering the types of mind/virtual-world these genes express. Thus the non-specialist user of genetic management software could, in principle, be shielded from the complex chemical minutiae of what is happening many virtual layers of complexity below, just as most PC users today wouldn't recognise machine code if it bit them on the nose.
Pessimists might argue that the opportunity for such life-transforming manipulations will be solely the privilege of a rich elite. This anxiety is (probably) misplaced. For the time-lag between the introduction of a new technology and its diffusion to the population at large has been progressively shrinking. It took perhaps 50 years to democratise the radio; some 20 years for the TV; around 10 years in the case of the PC; and even less for the mobile phone. With information-based products, the time-lag effectively collapses. Thus the gap between an expensive software release in Redmond and its availability to the population of Thailand is perhaps a few hours. Irritating bottlenecks notwithstanding, information is cheap. Technically, the "counterfeit" generic version is in no way inferior to the brand-name product. In a mature information-based society, "scarcity" is a far more elusive concept than in the era of material commodities. On this analysis, the resources of, for instance, tomorrow's domestic quantum computers may be harnessed anywhere and everywhere to more humanly empowering pursuits than the factoring of thousand-digit numbers that so excites contemporary cryptographic theorists. A good place to start will be simultaneously screening an unimaginable multitude of alternate histories of gene- and drug-combos in search of promising leads for one's personal development program. The friendliest, voice-activated, most visually compelling user-interfaces that creative designers can build may make seizing control of one's destiny from a legacy genome a less daunting challenge than it seems today. This doesn't mean we won't need all the help we can get in mastering the awesome software tools soon available to personalise our own genome and drug-regimen of choice. But choosing who and what we want to be should feel exhilarating rather than intimidating.
In this optimistic scenario, a product-pipeline of better, faster, cheaper and safer designer-drugs and drug-and-gene-combos should in principle be accessible to everyone within 15-20 years. Sustainable E-like empathogen-entactogens may become as familiar as aspirin; much safer in overdose; and far more ubiquitous. The design of E-like hugdrugs, lovedrugs and euphoriants, and perhaps later the genetic programming of E-like waking consciousness, could be just the beginning of a whole new genetic and chemical cornucopia for mature post-Darwinian life. For now, certainly, the prospect of a loved-up world reads like overheated science-fantasy. In practice, such predictions may prove too tame to be realistic.
The molecular machinery of magic
Lifelong ecstatic wonderpills and genetic self-mastery are at best some way off. So which ingredients of MDMA's primal magic are most worth mimicking pharmacologically in the near-term future? Preventing tolerance, promoting safety, and indefinitely extending duration are vital. Yet how desirable is inducing more or less euphoria, more or less calmness or behavioural activation, purer empathogenic or entactogenic action, and a greater or lesser hint of trippiness?
Pre-treatment studies with receptor antagonists indicate that dopamine D2 antagonists such as haloperidol (Haldol) attenuate MDMA's positive hedonic effects; 5-HT2A antagonists like ketanserin suppress MDMA's residual psychedelic activity; and SSRIs like citalopram (Celexa / Cipramil, the most selective of the SSRIs) diminish if not abolish the full spectrum of MDMA's psychoactivity. Drug discrimination studies performed on captive rodents may overlook certain subtleties of the MDMA experience. Intriguingly, in mice at least, the behavioural and physiological effects of MDMA are selectively antagonised by the plant alkaloid nantenine (9,10-methylenedioxy-1,2 dimethoxyaporphine). Nantenine is "antiserotonergic" and an alpha1-adrenoceptor antagonist; the effects of nantenine on ecstatic human subjects are unknown. But on present evidence, it's primarily a combination of enhanced oxytocin release and the interplay between the serotonergic and dopaminergic systems that underlies MDMA's discriminative stimulus effects/sublime magic.
The full story is complex and still poorly understood. As the user "comes up", serotonin released into the synaptic cleft activates multiple serotonin receptor subtypes (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7), and subpopulations (most notably, 5-HT1B, 5-HT2A and 5-HT2C). The hierarchy of their relative contributions to the subjective and behavioural effects of MDMA use may shift with increasing dosage and the course of the trip. Several of these serotonin receptor subtypes have functionally opposing roles, notably the effects of 5-HT1A and 5-HT2C receptor agonism on anxiety. As well as inducing a synaptic flood of serotonin, taking MDMA indirectly induces the release of extra dopamine in the mesolimbic reward centres. Activation of the serotonin 5-HT1B and 5-HT2A receptors leads to an increase in the vesicular release of dopamine. Dopamine levels are also increased by reuptake inhibition. In addition, dopamine synthesis is increased and turnover reduced. Increased synaptic availability of dopamine in turn inhibits glutamate-evoked firing in the nucleus accumbens. Dopamine released in the shell of the nucleus accumbens inhibits the firing of GABAergic medium spiny projection neurons. Inhibited excitability of the spiny projection neurons in the rostral shell of the nucleus accumbens - whether it's mediated by dopamine, glutamate antagonists or mu opioid agonists - is the neurological signature of euphoric bliss, whatever its guise.
On MDMA, there's much more going on as well. MDMA induces the release of noradrenaline (norepinephrine), and inhibits its reuptake. MDMA also triggers the enhanced release of acetylcholine in the striatum and prefrontal cortex via serotonin 5-HT4 and dopamine D(1) receptor mechanisms. Taking MDMA activates the poorly understood sigma(1) receptors, contributing to its locomotor stimulant action. The role of MDMA in activation the cannabinoid CB1 receptors to modulate its rewarding action is poorly understood. MDMA exerts (weak) binding to the alpha-2 adrenergic and histamine H1 receptors; this binding contributes in unknown degree to behavioural stimulation. Activation of the noradrenaline system causes an acute elevation of blood pressure. Additionally, taking MDMA increases plasma cortisol, prolactin, and dehydroepiandrosterone (DHEA) and aldosterone secretion. MDMA use alters the expression of several proteins involved in GABA transmission. To thicken the plot further, MDMA triggers the release of hypothalamic arginine-vasopressin and oxytocin (the "cuddle hormone"). These hormonal changes may influence some of MDMA's psychological effects. But the current consensus is that enhanced serotonin and dopamine release are crucial to the magic, even though they don't explain it.
The serotonin system is uniquely complex. A whistlestop tour can't do it justice. The existence of the serotonin molecule in Nature long predates the brain; serotonin is found in both the plant and animal kingdoms. However, the effects exerted by a neurotransmitter on the post-synaptic membrane aren't determined by the chemical itself, but rather by the structure of the post-synaptic receptor subtypes to which it binds. Our serotonin-producing neurons belong to a phylogenetically ancient neurotransmitter system. In the vertebrate CNS, serotonin-producing neurons regulate aggression, impulse-control, mood, anxiety, cognition, temperature, appetite, circadian rhythms, sexual activity, sleep, sensorimotor integration, sensitivity to pain, emotional resilience and romantic love. Serotonin entering the axonal vesicles is released over time in response to action potentials by exocytosis into the synaptic cleft, the narrow gap 10-20 nm across between pre- and post-synaptic neurons. Seven distinct families of serotonin neuronal receptors have been isolated; 14 sub-populations of G-protein-coupled receptors and one family of ligand-gated ion channels (the 5-HT3 receptor) have been cloned. Distribution, density and regulation of the serotonin receptors vary in different areas of the brain. So does both the affinity of serotonin for its different receptor subtypes and the effects of serotonin agonists on second-messenger systems. Only a few hundred thousand of the 100 billion or so neurons in the brain manufacture serotonin. The serotonergic cell bodies are confined to the raphé area in the brainstem, but their projections extend to almost all areas of the brain and spinal cord. Most notably for E-users, serotonergic projections innervate the dopaminergic nigrostriatal and mesocorticolimbic circuits. The serotonin system has co-evolved with dopaminergic projections in the course of primate evolution. Amongst many other roles, the serotonin system helps to regulate a lifetime spent in complex social hierarchies where more ancient fight-or-flight reactions have been offset by the need for an increasingly complex cognitive, emotional and behavioural response. This unique signalling complexity of the serotonin pathways and their multiple receptors ensures we can now be (un)happy in more ways than ever before.
The serotonin/5-hydroxytryptamine molecule itself is an indole amine synthesized from the essential amino acid L-tryptophan through the intermediate 5-hydroxytryptophan. Although some serotonin is present in the cytoplasm of serotonergic cell bodies and nerve terminals, most serotonin in the axonal terminals is sequestered in small membrane-bound sacs, i.e. the synaptic vesicles. This prevents the neurotransmitter from being metabolised by the enzyme MAO. Serotonin is metabolised, mainly by MAO-type A, into the inactive metabolite 5-hydroxyindoleacetic acid (5-HIAA). Numerous studies have shown self-destructive violence, aggression, poor impulse-control, reduced social status, suicide, and some types of depression are associated with low concentrations of cerebrospinal fluid 5-HIAA. Consequently, these conditions are often conceived as disorders of "low serotonin function". Firing of the serotonin neurons causes exocytosis, a rapid calcium-dependent process of neurotransmitter release. Depolarisation of the axon induces opening of voltage-sensitive calcium channels; the resultant calcium influx causes synaptic vesicles to fuse with the plasma membrane, where they empty their load of serotonin into the synaptic cleft. In the synapse, serotonin exerts an action on both pre- and post-synaptic receptor sites. Extracellular serotonin is then normally taken back up into the serotonergic neuron via the highly efficient presynaptic transport pump. The structure of the transporter protein determines how it couples ion gradients to substrate transport in ways that still need to be clarified.
Whatever the precise details, taking MDMA causes a remarkable role-reversal of normal transporter function. The MDMA molecule binds with high affinity to the serotonin transporter and enters the presynaptic axon terminal. Current theory suggests that MDMA causes serotonin release via a diffusion exchange mechanism involving the serotonin transporter, not by calcium-dependent exocytosis of the serotonin-containing secretory vesicles. MDMA taken up into the presynaptic terminal unbinds from the uptake transporter, triggering a reconfiguration of the transporter so it binds to serotonin inside the cytoplasm of the nerve terminal. The reconfigured transporter then reverse-pumps the newly-bound intracellular serotonin out of the cell, changes configuration again, dumps the serotonin into the extracellular space, and then takes up MDMA once more, repeating the process of depletion rather than recycling the neurotransmitter.
The ensuing flood of serotonin in the user's synapses sets the MDMA magic rolling. The neurotransmitter binds to multiple serotonin receptor subtypes. The subtypes play different excitatory and inhibitory roles. So which receptor subtypes are of most long-term therapeutic and social-recreational interest to a notional paradise-engineer? Like the proverbial drunkard who searches for his lost keys only under a lamp-post "because that's where the light is", investigators focus first on wherever they can probe most easily. The receptor-based account below will soon be superseded by something deeper. But it probably at least offers clues to the full story.
Serotonin 5-HT1 agonists, sometimes termed serenics, show pronounced anti-aggressive properties. Aggressive behaviour is modulated in by the 5-HT1B receptors in particular. The presynaptic 5-HT1B terminal autoreceptors form a vital part of a feedback mechanism regulating serotonin synthesis and release. Receptor knock-out mice lacking the 5-HT1B receptor are superficially normal in appearance, feeding patterns and breeding behaviour; but they are ferocious, and highly reactive. Such knockout mice are also unusually partial to alcohol and supersensitive to the effects of cocaine, though these traits may reflect a compensatory enhancement of the dopamine system rather than offer a direct pharmacological model of 5-HT1B receptor function. By contrast, 5-HT1B receptor agonists such as the drug anpirtoline exert "serenic" effects. In "animal models", 5-HT1B receptor agonists diminish alcohol-heightened aggression. Surprisingly, perhaps, there is substantial evidence to indicate that some endogenous serotonergic pathways normally activate rather than suppress motor output. Acute activation of 5-HT1B receptors is known to play a role in MDMA-induced locomotor activity: 5-HT1B agonists and MDMA show cross-tolerance, suggestive of a common mechanism of action. 5-HT1B antagonists restrain the hyperlocomotion that rodents and clubbers typically undergo on serotonin-releasers like MDMA. Perhaps with this crude behavioural measure in mind, some "unlicensed" psychonauts try combining a supposedly 5-HT1B-selective agonist such as the piperazine derivative TFMPP [1(3-trifluoromethylphenyl)piperazine monohydrochloride] with psychostimulants like 1-benzylpiperazine (BZP, "A2", "Frenzy", "Nemesis", "Flying Angel", "Altitude", etc) to try and replicate the acute effects of taking MDMA. The effect can indeed be E-like; but results are mixed. It is now known that TFMPP binds at multiple serotonin receptors with only limited selectivity. Taken in the absence of a dopaminergic psychostimulant, TFMPP does not feel akin to MDMA. Even combined with a dopaminergic, TFMPP's activation of the 5-HT2C receptors makes some users feel anxious. The MDMA effect is hard to emulate: MDMA is "a multifaceted jewel", not a cheap-and-cheerful euphoriant.
Some 1-benzylpiperazine users report that low-dose BZP is itself mildly E-like. So-called Party Pills, Social Tonics, Legal Party Drugs, Legal Herbal Highs and the like are marketed under numerous brand names, commonly mixed with various amino acids, vitamins, herbs and other agents designed to modulate the core BZP experience and minimise side-effects. Over 20 million such tablets and capsules have reportedly been sold in New Zealand, currently the world leader in BZP consumption and export. BZP use is now spreading world-wide. Consumption is mostly by clubbers seeking a safer legal alternative to MDMA or amphetamines. BZP is relatively safe if used in moderation. No fatality has yet been recorded from BZP use alone. Many BZP-based preparations contain black pepper, a tribute not to BZP's wholesome natural origins (it's synthetic), but to discourage thrill-seekers who might otherwise snort rather than swallow it. The Social Tonics Association of New Zealand (STANZ) has developed a Code of Practice to encourage responsible use. Inevitably, the FDA has now banned BZP on grounds of abuse potential. In 2002 the drug was made Schedule 1 and its US users criminalised. Sale in the UK was banned in 2007. BZP was originally (1944) synthesised, developed and manufactured by Wellcome as a potential anti-parasitic. Many piperazine drugs tend to paralyse intestinal parasites, allowing unwanted fauna to be flushed from the body. In the 1970s, BZP was investigated as an antidepressant. Used experimentally, BZP proved effective in reversing melancholic/retarded/hypersomnolent depression. The BZP analogue N-ben-zyl-piperazine-picolinyl fumarate was even briefly marketed in Hungary under the brand name Trelibet as an antidepressant, though no clinical trials have been conducted into its long-term efficacy. Development was halted after BZP was discovered to have amphetamine-like properties. Amphetamine, methamphetamine and BZP alike do indeed release dopamine from non-vesicular pools; and the subjective effects of high-dose BZP cross-generalise to amphetamines in drug discrimination studies. However, the subjective effects of BZP are more subtle than crude speed; BZP promotes the release and (to a lesser degree) inhibits the reuptake of the monoamine neurotransmitters dopamine, noradrenaline and serotonin with different relative potencies. BZP also acts as a relatively non-selective serotonin agonist. The affinity of BZP for the serotonin 5-HT2A receptor is quite low, so psychedelic effects are comparatively minor at sensible doses. Taking BZP doesn't give rise to the inner serenity, emotional release or extraordinarily empathetic compassion of MDMA; but nor is BZP a classic dopaminergic power-drug like amphetamine. Moderate behavioural activation, sensory enhancement and a low-key euphoria are typical. Dry mouth, appetite suppression and insomnia are common adverse side-effects. Unlike, say, cocaine, BZP use tends to be self-limiting, a critical advantage for a recreational drug. Yet subjects who hope to replicate the full richness of the MDMA experience will be disappointed.
There are further subtleties in the way of replicating MDMA's acute effects; and even more obstacles to sustaining the magic indefinitely. The serotonergic system has both 5-HT1B autoreceptors and post-synaptic 5-HT1B heteroreceptors; they play different functional roles. 5-HT1B receptors acting as autoreceptors regulate serotonin release via inhibitory feedback at the presynaptic terminals of serotonergic neurons; turnover and release of serotonin are typically increased under conditions of acute stress. 5-HT1B heteroreceptors are located on the terminals of nonserotonergic neurons. Thus 5-HT1B heteroreceptors regulate the release of other neurotransmitters. A single serotonin neuron can modulate different brain functions and multiple cellular targets in virtue of the thousands of non-synaptic varicosities on its axonal branches that project to multiple areas and neurotransmitter systems. 5-HT1B receptors within the ventral tegmental areas (VTA), for instance, function as heteroreceptors to inhibit GABA release. Since the GABA terminals in the VTA and substantia nigra exert a tonic inhibitory influence on dopamine function, inhibition of GABA by inhibitory 5-HT1B heteroreceptors leads to the disinhibition of dopamine activity. Thus agents acting directly or indirectly as 5-HT1B agonists can cause the release of dopamine in the striatum and nucleus accumbens. Indirectly again, dopamine release is also regulated by 5-HT1B heteroreceptors within the glutamatergic hippocampo-accumbens pathways. Regulation of 5-HT1B receptor function itself is under the control of 5-HT-moduline, an endogenous tetrapeptide that controls 5-HT1B receptor efficacy. 5-HT-moduline is a so-called allosteric modulator. Allosteric modulators bind to a different binding site from the natural agonist and can, potentially, circumvent the development of tolerance. 5-HT-moduline is released from adrenal medulla in response to acute stress. 5-HT-moduline plays a pivotal role in synchronising the serotonergic signalling activity of the different terminals of individual neurons, coordinating their effects on a variety of different cerebral functions. Rationally designed synthetic drugs that recognize the 5-HT-moduline binding-site on the 5-HT1B receptors, and act on the 5-HT1B receptors as allosteric modulators themselves, may potentially exert long-term serenic, anxiolytic and mood-brightening effects by increasing serotonin release.
In general, however, care must be taken in describing serotonin 5-HT1 agonists as "serenics", even if such agents induce a syndrome outwardly suggestive of inner tranquillity. The demeanour that an animal exhibits after "serenic" administration may indeed be submissive, passive and timid - in contrast to the fierce, assertive and aggressive behaviour of 5-HT1B knockouts. Yet "serenity" tends to connote an inner E-like peace that may be lacking - and not just in the unfortunate laboratory rodent. In fact some so-called "serenics" may enhance fear/anxiety reactions: it's only their use in combination with dopamine-releasing euphoriants that makes such agents especially interesting to the psychonaut. Indeed supersensitive 5-HT1B autoreceptors are implicated in depression and obsessive compulsive disorder. By introducing extra copies of the gene for 5-HT1B receptors into serotonin neurons, researchers can breed passive and depressive rats that show signs of abject misery [i.e. "learned helplessness" and "behavioural despair"]. The syndrome of learned helplessness is associated with excess production of 5-HT1B receptors that are churned out in greater profusion by the depressive brain. This isn't to deny that 5-HT1B agonists may have therapeutic potential, whether in bipolar disorder, autism, alcoholism or disorders of impulse-control and aggression. Thus the triptans, serotonin 5-HT1B/1D receptor agonists, are clinically effective for treating migraines; they can also curb aggression. But 5-HT1B antagonists and inverse agonists such as SB-236057-A are under investigation for possible clinical use as long-term and relatively fast-acting antidepressants. Acute 5-HT1B autoreceptor blockade can increase serotonin release. Cognitive function is affected by their use too. Whereas 5-HT1B agonists may adversely affect memory via inhibition of acetylcholine release in the hippocampus, antagonists and inverse agonists of the 5-HT1B receptor can improve the consolidation of learning. This simplified outline of the neurobehavioural role of a single family of serotonin receptor subtype illustrates how inducing lifelong E-like states - as distinct from "mere" raw bliss - is going to pose a formidable technical challenge. In this case, the possible existence of multiple subpopulations of 5-HT1B autoreceptors and heteroreceptors makes inadequate selectivity of ligands even more of a problem, especially for seekers of precision-tools rather than chemical coshes.
Whereas serotonin 5-HT1B receptor knockout animals are aggressive by nature, 5-HT1A knockouts are timid, anxiety-ridden creatures. Whereas serotonin 5-HT1B receptors are found mainly on terminal processes, 5-HT1A receptors are located solely on serotonergic nerve cell bodies within the dorsal raphé nucleus. Intriguingly, 5-HT1A receptor density is reported to be inversely correlated with susceptibility to spiritual experience, opening up the possibility of genetically amplifying our capacity for spirituality beyond anything humanly accessible today: it may be premature to assume that our descendants will be secular rationalists. Density of the 5-HT1A autoreceptors is also inversely correlated with the reactivity of the amygdala to threatening stimuli. However, the role of the 5-HT1A receptors in MDMA's acute subjective effects still isn't clear. Pretreatment with a serotonin (5-HT1A) receptor antagonist apparently reduces MDMA's pro-social effect, in rats at least. Taken over a prolonged period, selective 5-HT1A receptor agonists typically exert a delayed-onset anxiolytic as well as (sometimes) a mood-brightening activity. Their (modest) therapeutic efficacy relies on an adaptive neuronal response. Acute activation of the presynaptic 5-HT1A receptor on the raphé nuclei tends to reduce both the rate of firing of serotonin neurons and the corresponding release of serotonin from the nerve terminals; chronic activation causes the receptors to desensitise, leading serotonergic neuronal activity to rebound. Clinically, buspirone (Buspar), a 5-HT1A partial agonist, is licensed for generalised anxiety disorder. Similar agents like gepirone (Ariza), flesinoxan, tandospirone and ipsapirone are under investigation. Alas taking them doesn't remotely engender the extraordinary sense of inner peace induced by MDMA. In rats at least, 5-HT1A agonists facilitate male sexual behaviour, hypotension, increased food intake and produce hypothermia, none of which are prominent sequelae of MDMA use. In general, 5-HT1A agonists are well tolerated. But they may also on occasion induce dizziness, nausea, and headaches, probably linked to their postsynaptic receptor action rather than presynaptic anxiolytic effect. Buspirone itself is also a dopamine D2 antagonist, albeit a weak one. This may explain why it's never been wildly popular with patients. It's also very slow to work. Gepirone, on the other hand, allegedly lacks significant activity at the dopamine D2 receptors. Gepirone acts as an agonist at the presynaptic 5-HT1A receptors and a partial agonist at the post-synaptic 5-HT1A receptors. Hopefully, gepirone will prove a clinically useful anxiolytic and antidepressant. However, though 5-HT1A antagonists reduce discrimination of MDMA in animal models, the role of 5-HT1A receptor activation in MDMA's effects needs elucidation via more first-person experimental studies.
Recent research from Sydney University neuropharmacologist Iain McGregor suggests that post-synaptic serotonin 5-HT1A receptors contribute to MDMA's acute pro-social action via the enhanced release of oxytocin. Oxytocin in turn reduces activity and weakens connections in the fear-processing circuitry of the amygdala. MDMA activates post-synaptic 5-HT1A receptors of the paraventricular nucleus and supraoptic nucleus of the hypothalamus. The paraventricular nucleus and supraoptic nucleus contain oxytocin neurons. Oxytocin is a nine amino-acid peptide hormone and neurotransmitter that promotes pair-bonding, trust and social recognition. Commercially, oxytocin is marketed by Verolabs as a spray: so-called trust-in-a-bottle: "Liquid Trust Spray - the first Oxytocin product, formulated to enhance people's trust in you!"[sic]. In future, MDMA analogues may conceivably be marketed with similar restraint. MDMA typically causes its users to trust each other to an exceptional degree, confiding intimate personal feelings and secrets they would never otherwise share. Such drug-induced intimacy may partly be mediated by an increased release of oxytocin via MDMA-activated 5-HT1A receptors. Co-administration of MDMA and an oxytocin antagonist would test this hypothesis in humans. There are methodological problems with the use of rats as test subjects in this context; but the evidence is suggestive.
The MDMA molecule, especially the dextrorotatory "+" isomer, has only a low affinity for the serotonin 5-HT2 receptor. This is why taking the drug within the normal dose-range typically induces only minor perceptual changes. If prompted, many Ecstasy users report altered time perception, but any visual distortions are usually mild: the N-methyl group of the MDMA molecule prevents it from fitting as comfortably into the 5-HT2A receptor as does the trippier (-)-MDA enantiomer of its structural parent. Experiments with human as well as non-human animals show a correlation between a drug's psychedelic potency and 5-HT2A receptor binding affinity. Activation of the 5-HT2A receptors is a prerequisite of the "classic" hallucinogenic effects exerted by tryptamine psychedelics such as LSD and phenethylamine psychedelics like DOM. Conversely, 5-HT2A receptor inverse agonists act as antipsychotics. Despite the low affinity of MDMA for the 5-HT2 receptor, pharmacological blockade or genetic knock-out of the 5-HT2B receptor abolishes MDMA-induced hyperlocomotion and serotonin release in the nucleus accumbens and ventral tegmental area of the brain.
None of this neurobabble should disguise the fact that psychedelia is still scientifically uncharted. It's often too weirdly exotic for words. Materialistic neuroscience has failed to close the ontological gulf between neural porridge and consciousness - whether "ordinary" or "altered" states. Some psychonauts, understandably enough, feel the neurobabblers have lost the plot. Most of today's storytelling about altered states and the chemistry of mind will doubtless seem no less archaic to our descendants than the Greek humoral psychology of classical antiquity strikes the contemporary molecular biologist. Yet fortunately for the engineering purposes of inducing sustainable E-like bliss, we need manufacture only the sufficient neural conditions for beautiful states of consciousness. We don't need a deep understanding of how and why consciousness is generated (or alternatively, some philosophers allege, its fundamental immanence in the world). We can guess even less about the possible altered states of consciousness of our redesigned successors. We don't know whether the "explanatory gap" between the physical facts and phenomenal mind can ever be closed. But either way, our emotionally invincible descendants should be able to explore entheogens, and map out even the most outlandish reaches of psychedelia, in safety. Unlike us, our genetically enriched descendants may revel in the assurance that bad trips are inconceivable, and psychological damage is impossible. This is because their obnoxious molecular substrates will have been edited out.
Alas our own less robust minds are psychologically vulnerable to even "physically" harmless psychedelics that aren't also euphoriants. Dual-action dopamine- and serotonin-releasers like MDMA are the latter, though they aren't always harmless. With MDMA, as with so many psychoactive drugs, very often "less is more". This piety is easy to intone but hard to practise, especially when taking fast-onset euphoriants. The lucidity of the entactogenic effect of MDMA may be especially pronounced at low-to-moderate dosages. "Optimal" dosage of psychotropic agents taken for "non-approved" purposes is most often empirically determined by the user investigating what level induces maximal enjoyment. Yet the effects of lower, "sub-optimal" dosages that more subtly modulate consciousness may be of greater value for facilitating personal growth. Low-to-moderate dosage E-experience may be easier to integrate into the rest of one's E-less life. Nonetheless at higher, quite possibly neurotoxic doses of 200mg or so, MDMA can itself sometimes deliver psychedelic euphoria, entheogenic rapture, and some very interesting exotica indeed. Alas the unique effects of such doses [and likewise higher doses of other stellar phenethylamines] cannot safely be investigated in depth until the neurotoxicity of MDMA's metabolites and/or toxic free radicals can be prevented.
In the meantime, if the user desires a completely clear sensorium, then perceptual alterations might seem eliminable altogether, in principle, by taking only the (+)-MDMA enantiomer rather than the standard racemate. Sadly, pure (+)-MDMA is scarce; it's also hard to prepare at home. Thus one unintended consequence of scheduling MDMA has been to widen youthful exposure to psychedelia, albeit psychedelia in its warmest and most gentle introductory guise. (-)-MDMA at normal doses is only minimally active at the "psychedelic" 5-HT2A receptor owing to its (comparatively) bulky methyl group. By contrast, MDA (which lacks it) is an all-in-one cocktail that can be hallucinogenic as well as empathetic and slightly speedy.
Alternatively, if uncomplicated perceptual clarity is sought then a 5-HT2 antagonist such as ketanserin or the 5-HT2A selective MDL-11939 might help preserve total lucidity. 5-HT2A antagonists have the additional advantage of preventing MDMA-induced hyperthermia that exacerbates toxicity. Neurotoxic hydroxyl radical formation is temperature-mediated; conversely, hypothermia-inducing agents enhance neuroprotection.
However, there are complications. Stimulation of the serotonin 5-HT2A receptors contributes to the rewarding effects of MDMA, or at least plays a permissive role in dopamine release. So trying to eliminate perceptual alterations completely while retaining the full-blooded E-magic may be difficult. MDMA is often reckoned a "serotonergic" drug. Compared to amphetamine this is true: MDMA's affinity for the serotonin transporter is greater, and its ratio of serotonin to dopamine release is higher, than amphetamine. Even MDMA's extra release of dopamine partly depends on its activation of the 5-HT2A receptors. But serotonin-releasing agents [e.g. the halogenated amphetamine appetite-suppressant fenfluramine (Pondimin)], taken on their own, aren't notably rewarding or entactogenic/empathetic, at least at ordinary dosages. The enhanced release and reuptake inhibition of dopamine is essential to MDMA's tendency to promote blissful well-being and to colour its entactogenic-empathetic effect.
Convergent strands of evidence indicate that dopamine release is critical to the MDMA magic. Dopaminergic activity in the brain and motor behaviour may be crudely interpreted as under the inhibitory control of the serotonin system. Yet the multiple serotonin pathways play functionally different roles. According to one hypothesis, the extra serotonin released by MDMA stimulates 5-HT2A receptors located on inhibitory gamma-aminobutyric acid (GABA) striatonigral neurons. VTA dopaminergic neurons in the brain's reward centres are under continuous inhibition by GABA. Stimulation of the 5-HT2A receptors inhibits these GABA neurons, thereby allowing the disinhibition of dopamine biosynthesis. Post-E levels of dopamine in the mesolimbic reward circuitry are far higher than would be explained by MDMA's relatively weak additional release of dopamine via the uptake carrier.
Animal drug discrimination studies, and the human behavioural evidence, tend to support this dopaminergic account. Although some MDMA users prefer reflective tranquillity and intimate group hug-ins, many loved-up clubbers opt to dance for hours at raves - a form of hyperlocomotion one would expect from Peruvian marching-powder rather than a serotonergic agent.
However, this account is still simplistic. The release of serotonin following an MDMA-induced reversal of the reuptake pump results in a stimulation of the 5-HT1B receptors and, at higher doses, increasingly of the 5-HT2A receptors as well. Such receptor stimulation can trigger marked hyperactivity, especially in young MDMA users who rave. At lower doses, MDMA-induced locomotor activity is caused mainly by the released serotonin's preferential activation of the 5-HT1B receptor. This is because serotonin has a somewhat higher affinity for the 5-HT1 receptors than the 5-HT2 receptors. The greater flood of serotonin in the synapses triggered by higher doses of MDMA promotes locomotor activity via 5-HT2A receptor-mediated dopamine stimulation as well. To complicate matters, MDMA may itself bind, albeit weakly, to the 5-HT2A receptor. A further complicating factor is that MDMA-induced release of serotonin stimulates the 5-HT2C receptors. Activation of the 5-HT2C receptors serves to mask expression of MDMA-induced hyperactivity, sometimes evidently more effectively than others. The various subpopulations of 5-HT2C receptor located on GABAergic neurons in the ventral tegmental area and the substantia nigra tend to exert a tonic inhibitory influence over the mesolimbic dopamine system. Thus 5-HT2C receptors tonically inhibit dopamine release in the nucleus accumbens, mostly it seems in virtue of their constitutive activity i.e. entering the activated receptor state in the absence of an agonist. Other things being equal, activation of 5-HT2C receptors is anxiogenic, demotivating and generally unpleasant. Certainly the stimulant effects of MDMA are greatly enhanced following treatment with a 5-HT2C antagonist. Sustained antagonism of the 5-HT2C receptors might well we harnessed to intensify the hedonic properties of long-lasting E-like consciousness. Less speculatively, 5-HT2C antagonists such as agomelatine (Valdoxan) are under investigation as potential clinical antidepressants.
As usual, there are complications: all 5-HT2C receptors are not the same. Numerous 5-HT2C receptor isoforms are produced as a result of RNA editing, and their individual roles in modulating the MDMA effect aren't properly understood. In general, the receptor story illustrates at the molecular level that being blissful isn't the same as being blissed out. To sustain empathetic love, simply banishing all capacity for social anxiety isn't going to work. Specific and selective 5-HT2C receptor antagonism may well prove a worthwhile goal; but it's too early to say what the MDMA experience may gain or lose in consequence, whether socially or subjectively. Empathy entails caring about others, not lacking a care in the world. Thus the MDMA-induced disinhibition from social anxiety, and the lowering of psychological defensive barriers, is radically distinct from the sort of anxiolysis induced by SSRIs or the benzodiazepines - or indeed by alcohol or opiates. With none of these drugs or drug categories is a reduction in the user's social anxiety matched by an E-like upwelling of empathy or sensitivity to the feelings of others - in fact quite the reverse. There are subtleties of the MDMA experience that haven't yet been explored.
If acute serotonin-mediated enhanced dopamine-release is indeed essential to the magic of MDMA, then a wide range of safe long-acting dopaminergics are already on offer to augment any hypothetical subtype-selective "serotonergic" therapies. Compared to our descendants, we're probably all anhedonic. So some form of dopaminergic augmentation is a therapeutic step in the right direction. "Dual-deficit" models of everyday E-less malaise are plausible; and they naturally invite dual-action remedies. Clearly, inhibition of glutamate-evoked firing in the nucleus accumbens is an ingredient of the E-magic: it is known that firing-inhibition depends on both dopamine and serotonin release; and this process is mediated by both dopamine and serotonin receptors. But beyond these superficial generalities, working out how to replicate sustainably at the molecular level the precise neurochemical signature of peak experiences will be hard. Until the dawning of the era of wholesale genomic rewrites and true designer babies, using a cocktail of subtype selective serotonin agonists and gentle dopaminergic psychostimulants still looks like the easiest way to mimic and enhance the entactogenic-empathogenic effect induced by MDMA-like compounds. However, there are many pitfalls in choosing the right dopaminergic for the job.
In contrast with intracranial electrical stimulation, a direct chemical assault on the hedonic treadmill rarely works. This failure is witnessed by the unsatisfying and usually counterproductive effects of using catecholamine-depleting psychostimulants. Darwinian-era mood and motivation is regulated via a multitude of indirect mechanisms of feedback-inhibition. So it's worth reviewing how and why the substrates of human well-being are held in check; and what can be done about it. First, an unavoidably fast-and-furious tour of the dopamine system is in order. The CNS has three main dopaminergic pathways. They regulate movement, hormonal secretion, and emotion. Each projects from dopaminergic cell groups in the midbrain. 1) The nigrostriatal pathways extend from the substantia nigra pars compacta to the striatum. This pathway is critical to the control of involuntary motor movement; its dysfunction is implicated in the tremor, rigidity and akinesia of the "dopamine deficiency disorder" Parkinson's disease, and several other neuropsychiatric disorders such as Tourette's Syndrome. 2) The tuberoinfundibular system extends from the hypothalamus to the pituitary gland. It's involved in prolactin- and growth hormone-secretion, and the regulation of lactation and fertility. 3) The mesocorticolimbic pathway extends from the ventral tegmental area to the nucleus accumbens and the medial prefrontal cortex. The mesocorticolimbic system is central to emotion, motivation, willed action and, more subtly, the modulation of thought-processes. In crude terms again, dopamine is critical to sensorimotor integration; appetitive behaviour of all kinds; the capacity to switch from one course of behaviour to another; and the orchestration and activation of the motor output system. Dopamine has also traditionally been described as the brain's "pleasure chemical", cueing potentially (Darwinian) fitness-enhancing stimuli so they can acquire control over an organism's behaviour. Certainly, consistent with the dopamine theory of reward, electrically or pharmacologically stimulating microcircuits in the rostromedial shell of the nucleus accumbens produces intense pleasure in the absence of any goal-seeking behaviour. But this formulation can be misleading. The mesolimbic dopamine system mediates "wanting" more than "liking"; and its drug-induced or electrical stimulation may increase incentive-salience rather than the raw intensity of pleasure itself. Dopaminergic neurotransmission is critical to incentive-motivation and all forms of purposeful behaviour. Dopamine levels tend to rise if one is anticipating a rewarding event; and levels then tend to fall if the anticipated reward fails to materialise. Couched in the language of psychology rather than neuroscience, enhanced dopamine release in the pleasure centres imparts a sense of urgency, significance and a feeling of things-to-be-done. The molecular substrates of pure pleasure are still elusive.
At the cellular level, the dopamine system doesn't quite rival the molecular, pharmacological and functional diversity of the serotonin system; but the two "classic" types of dopamine receptor (D1-like and D2-like receptors) have several subtypes and alternate splice-forms. Further, the number of different messenger RNA and dopamine binding sites substantially exceeds the five dopamine receptor genes of the human genome, a diversity that reflects the genetic polymorphism and alternative splicing events in normal dopamine gene-expression. However, each type of dopamine receptor belongs to the superfamily of G-protein-coupled receptors that activates or inhibits different forms of adenylyl cyclase inside the cell. Intriguingly, the presence or absence of variant alleles of dopamine receptor subtypes and their signal-transduction mechanisms is correlated with variants of human behaviour and personality. For example, individuals with genotypes containing the seven-repeat allele of the dopamine D4 16-amino acid repeat polymorphism tend to exhibit the personality trait of "novelty-seeking". This trait is characterised by a tendency to impulsiveness, risk-taking, exploration, excitability, and an optimistic mood, though alas not a loving, E-like temperament. For better or worse, within a few decades prospective parents will be able to select such alleles and their rationally redesigned enhancements when choosing the parameters of their future offspring. Such naturally loved-up kids may prove more easily adorable than today's Darwinian default-models.
Like the other catecholamine neurotransmitters, dopamine itself is synthesised from the non-essential amino acid L-tyrosine. L-tyrosine is transported across the blood-brain barrier into the dopaminergic nerve cell. L-tyrosine is converted to L-dopa by the enzyme tyrosine hydroxylase. L-dopa is then rapidly converted to dopamine by L-amino acid decarboxylase. Next dopamine is sequestered in synaptic vesicles by a dopamine transporter. At the synapse, the dopamine nerve terminal displays high-affinity uptake sites. They rapidly terminate the action of the neurotransmitter on the receptors if it isn't metabolised by the MAO or COMT enzymes. Depending on concentration gradient, the dopamine carrier can transport dopamine back into the nerve cell, recycling it as normal, or alternatively, after a user has taken a classic amphetamine, the carrier can transport dopamine from the cell terminals into the synaptic cleft. In common with amphetamine, MDMA inhibits the neuronal reuptake of dopamine, albeit more weakly than MDA. Further, increased post-E administration activity of the serotonin 5-HT1B and 5-HT2A receptors causes the dopaminergic neurons themselves to fire more rapidly. This higher impulse-frequency causes increased dopamine-release via exocytosis of the dopamine-containing vesicles in the normal manner.
So what leaves so many "normal" Darwinian people - who are neither clinically depressed nor loved-up on MDMA - comparatively anhedonic and hypodopaminergic? The dopamine neurotransmitter is under powerful homeostatic control. So is the density and signal-transduction efficiency of the receptors to which it binds. Feedback-inhibition of dopamine synthesis, dopamine release and spontaneous action-potential generation in dopamine-producing cells is modulated by a variety of functionally distinct dopamine autoreceptors that regulate membrane excitability. The dopamine neurotransmitter itself functions as an end-product inhibitor of tyrosine hydroxylase, the rate-limiting step in dopamine production. Dopamine plays this role by competing with a tetrahydrobiopterin co-factor for a binding site on the enzyme. Dopamine synthesis is also modulated by the rate of impulse-flow from the nigrostriatal pathway. In addition, presynaptic dopamine receptors modulate the rate of tyrosine hydroxylation; and most mesolimbic dopamine neurons possess cholecystokinin-autoreceptors and neurotensin-autoreceptors that regulate dopamine function as well. Indeed activity of the mesocorticolimbic dopamine system is regulated by multiple neuronal pathways containing different neurotransmitters, notably serotonin, opioids, GABA and glutamate. Precisely what dopamine actually does in the all-important dopamine-sensitive shell of the nucleus accumbens is unclear. The main effect of its release seems to be the inhibition of the GABAergic medium spiny projection neurons (MSNs). These neurons come in two types. One subtype expresses dopamine D2 receptors and enkephalin. This sort of GABAergic medium spiny cell projects from the nucleus accumbens to the ventral pallidum. It is activated by "reward stimulation" of the ventral tegmental area. The other subtype of GABAergic medium spiny projection neuron co-expresses substance P, dynorphin and dopamine D1 receptors. This subtype projects directly back to the ventral tegmental area. It regulates motivation and pleasure, or our deficit thereof.
So how can this cruel and complex web of inhibitory feedback mechanisms best be modified? If our aim were pure-and-simple cloud nine euphoria, then better drugs to decrease glutamate and GABA currents in the critical medium spiny neurons of the nucleus accumbens might be adequate - at least until new genes and gene networks can be more readily inserted in the genome, and the regulation of old ones improved. But well-controlled, high-functioning euphoria is more elusive than mind-blowing rapture. Crude "natural" interventions to enrich dopamine function aren't effective. For instance, some psychonauts, clubbers and alternative therapists alike have explored taking free-form amino acid supplements of L-tyrosine and L-phenylalanine in a bid to boost native dopamine levels or reanimate a drug-frazzled brain. But tyrosine hydroxylase is normally saturated. So unlike tryptophan-loading and/or 5-HTP-loading to increase neural levels of serotonin production, this "dopaminergic" precursor strategy typically doesn't work. On the other hand, taking L-dopa does increase synaptic dopamine levels. This is especially so when L-dopa is combined (as in Sinemet for Parkinsonians) with a peripheral decarboxylase inhibitor such as carbidopa to prevent its metabolism outside the brain, At least for a minority of "normal" subjects, taking L-dopa can be an effective motivator, libido-enhancer and mood-brightener. In a more controlled setting, rodents engineered so they can't synthesize dopamine initially develop quite normally, only to die miserably a few weeks after birth following a failure to eat, drink or do very much in this world at all. Yet when such dopamine knock-out mice are abundantly maintained on L-dopa, they can flourish. Indeed L-dopa-maintained dopamine knock-out mice become hyperactive and sexually vigorous. This manipulation has not yet been attempted in dopamine knock-out humans. Augmentation should in any case be tried only cautiously and in controlled-release preparations (e.g. Sinemet SR) since high levels of L-dopa may increase oxidative stress. Whatever the mechanism, simply increasing raw dopamine levels per se is not enough. For instance, an agent such as alpha-methylparatyrosine that inhibits tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis, might be expected to produce a state of melancholic depression; but in non-depressives it doesn't reliably do so. This complicates any simplistic catecholamine-depletion theory of retarded depression. Nevertheless, dopamine-releasing agents demonstrably tend to induce euphoria. By contrast, dopamine receptor antagonists like haloperidol are dulling and dysphoric. All the classical dopamine D2-blocking neuroleptics blunt will-power and flatten emotion. Administering dopamine D2-blockers tends to induce apathy and anhedonia, and ruins the MDMA magic. Nasty but instructive, such magic-prevention experiments are an important pointer to what's needed to sustain the MDMA spectrum of consciousness. It's known that stimulation of the dopamine D2-like receptor causes an increase in phosphatidylinositol hydrolysis by activating enzyme phospholipase C. Enhanced phosphatidylinositol hydrolysis is implicated in euphoric mania. Conversely, the lithium used to treat "uncontrolled" euphoria inhibits the phosphatidylinositol second messenger system and darkens mood in nondepressed "euthymic" people. Understanding the principles behind the pharmacological induction of controllable non-stop euphoria will be a first step on the route to designing lifelong variations of the subtler forms of magic.
In the meantime, dopamine antagonists like amisulpride (Solian) can be used at low doses preferentially to antagonise the synthesis-, release- and impulse-modulating presynaptic dopamine D2/D3 autoreceptors. Thus a regimen of low-dose amisulpride may potentially enhance dopamine release and boost mood and motivation, whereas many dopamine reuptake inhibitors [e.g. vanoxerine, bupropion, nomifensine] "adaptively" diminish the neuronal release of dopamine over time, even though their action on reuptake inhibition increases the neurotransmitter's synaptic availability. Unfortunately, pre-treatment with high doses of dopamine reuptake inhibitors blunts MDMA-induced release of dopamine, though not to the same degree as SSRIs blunt MDMA-induced release of serotonin. Other crude strategies to augment dopamine function involve taking dopaminergic agents such as the dopamine agonists pergolide (Permax) and bromocriptine (Parlodel); the potent, pro-sexual, long-acting D2 agonist cabergoline (Dostinex); selective D2/D3 agonists such as pramipexole (Mirapex) or ropinirole (Requip); catechol-o-methyltransferase (COMT) inhibitors such as tolcapone (Tasmar); selective MAO-B inhibitors such as selegiline (Eldepryl) or rasagiline (Azilect); adenosine 2A receptor antagonists; and centrally active nicotinic receptor agonists. Oral, centrally-active dopaminergic "pro-drugs" with higher bioavailability and fewer adverse side-effects are also under investigation. But there are obvious problems. For instance, dopamine-release promoting agents, if fast-acting and taken in the absence of anything subtype selectively "serotonergic", may not induce serenely motivated well-being as distinct from compulsive pleasure-seeking, thought disturbances or manic excitement. Any tendency to cause uncontrolled dose-escalation is likely to cause toxicity, florid psychoses and abuse. Regrettably, these worries about the "abuse-potential" of psychostimulants frequently generalise in mainstream wisdom to an unwarranted fear of all "dopaminergic" antidepressants/mood-brighteners.
This taboo against "excessive" well-being can have serious medical consequences. Even victims of melancholic or retarded depression are widely denied access to clinically effective catecholaminergic antidepressants. This is one reason why so many remain depressed or "partial responders"; another is opiophobia. MDMA itself rapidly banishes all kinds of depression, albeit not for long. In spite of its relatively powerful indirect dopaminergic activity, MDMA is sometimes likened in the media to the much more commonly prescribed selective serotonin reuptake inhibitors; fluoxetine (Prozac) was the first and most famous SSRI. In reality there are profound differences between MDMA, the SSRIs and other "serotonergic" antidepressants. Like an SSRI, MDMA occupies the serotonin transporter and prevents serotonin from binding, increasing its availability in the synapse. However, MDMA is small enough to be taken up by the serotonin reuptake transporter into the serotonergic cell. The serotonin transporter pulls the MDMA molecule up into the axon, where its release from the transporter allows the transporter to bind to intracellular cytoplasmic serotonin, which it releases into the synapse before taking back more MDMA into the terminal. Quite aside from their different molecular mechanisms of action, however, there are striking differences in subjective effect between MDMA and "serotonergic" centrally active psychiatric medicines. Clinically-licensed SSRIs [fluoxetine/Prozac; sertraline/Zoloft; fluvoxamine/Luvox; paroxetine/Paxil; and citalopram/Celexa] may make a small minority of people feel durably "better than well". More typically, SSRIs are mood-blunters and even, for some people, psychic anaesthetisers. SSRIs commonly make those who take them more resilient and less anxious. But they don't promote depth of feeling, intellectual dynamism or clarity of thought. SSRIs can also diminish the intensity of love. MDMA, by contrast, is a veritable love-potion, what Claudio Naranjo aptly christened a "feeling intensifier". On MDMA, emotions are heightened as well as enriched. Compared to loved-up ecstatics on MDMA, the rest of us have the emotional intensity of zombies; and zombies have no real insight into what they're lacking, even if some of us can talk as though we do. Ironically, at a time when the loss of personal liberty entailed by prohibitionist drug laws is justified by the societal costs of illicit drug-taking, "psychiatric" drugs are clinically prescribed by physicians regardless of the likely effect of a medication on the personal relationships of the patient. SSRIs, by enhancing the user's emotional self-sufficiency, can either save marital relationships or wreck them. By reducing "neediness", SSRIs also diminish what today passes for love. SSRIs are prone to impair romantic ardour as well as libido. One technical (and ideological) challenge of the pharmacogenomic revolution in prospect at the interface between genetics and drug-design will be to investigate how the emotional honesty and extraordinary depth of feeling induced by MDMA can be sustained over a period of months, years and decades rather than for two-hour bursts.
There are further complications to overcome if any bid to replicate and sustain full-spectrum E-like consciousness is to succeed. MDMA triggers the release of the neurotransmitter acetylcholine via a histaminergic H1 mechanism. MDMA is also a weak agonist of the acetylcholine muscarinic M1 receptors. MDMA's modest cholinergic activity may contribute to the exquisite lucidity of consciousness characteristic of pure MDMA taken in a therapeutic setting. For the cholinergic system is vital to memory, higher thought-processes and verbal fluency. Cholinergics such as piracetam (Nootropil) are used as nootropics or "smart drugs"; and acetylcholinesterase inhibitors like galantamine (Reminyl), rivastigmine (Exelon), tacrine (Cognex) and donepezil (Aricept) are used as palliative treatments of Alzheimer's disease. Acetylcholine-release and muscarinic receptor activation probably play no direct role in the rewarding hedonic effects of MDMA. Yet their subtle contribution to the texture of the magic can't be discounted. "Dumb-drug" antimuscarinic agents commonly induce mild euphoria via their indirect enhancement of dopamine function. Their mood-brightening effect stands in contrast to many cholinergic (e.g. muscarinic M4 receptor) agonists and cholinesterase inhibitors which have a tendency to subdue mood. A wide range of cholinergics is now under development for the palliative treatment of Alzheimer's disease, a progressive neurodegenerative disorder characterised by profound cholinergic deficits. Some depressives, however, may actually benefit from the antimuscarinic anticholinergic effects that more intellectually fastidious clinicians would call an adverse side-effect of the older tricyclics. While a great many depressed people report intellectual sluggishness and poverty of thought, other melancholic and introspective depressives endure "hypercholinergic frenzy", possibly owing to dysregulation of the cholinergic-adrenergic axis. Sadly, innumerable depressives among life's walking wounded today find the examined life scarcely worth living: they cope with life only by "just getting on with it". By contrast, MDMA allows introspection to become insightful and enjoyable even to the naturally angst-ridden. On MDMA, both philosophising and emotional self-honesty can be illuminating and fun. It's a shame that such self-insight can't more readily be prolonged.
Another enigma is the role of DHEA. MDMA causes a rise in the adrenal corticosteroid dehydroepiandrosterone (DHEA). DHEA is the precursor to testosterone, progesterone, estradiol and other steroids. The rise and peak physiological values of DHEA between around 1 to 2½ hours post-MDMA administration is correlated with user-reported euphoria, though DHEA's precise contribution to the mood-elevation is unclear. In general, levels of DHEA decline with age after early adulthood. Long-term supplementation with DHEA seems to have beneficial effect on libido, immune function and some forms of cognition. However, in spite of a wealth of research, no firm conclusions have yet been reached on the advisability of taking DHEA supplements, or an optimal dosage if taken. Nor is it known what role enhanced DHEA might play in sustaining enriched quality of life over the longer term. Taken on its own, DHEA may brighten mood; but it's scarcely an E-like effect.
One unwanted effect of MDMA, especially when taken at higher doses, is its tendency inhibit to tryptophan hydroxylase by triggering a rapid oxidation of the enzyme's sulphydryl sites. Tryptophan hydroxylase is the rate-limiting enzyme in serotonin synthesis. Even though the acute functional loss of tryptophan hydroxylase in the cell terminal is reversible, the axon's vulnerability to oxidative stress is increased. In order sustainably to enhance our capacity for empathetic bliss, and certainly to prevent any functional serotonergic deficit, tryptophan hydroxylase function must be enhanced, not inhibited. However, to date no stimulator (or inhibitor) of the biosynthesis of serotonin has been commercially marketed. Interestingly, the use of interventions to increase the biosynthesis of serotonin prior to MDMA use tends to trigger an increased synaptic release of dopamine, thereby enhancing the user's euphoria. Unfortunately, increased serotonin synthesis also aggravates post-E neurotoxicity. The two mechanisms are separable in principle. In the meantime, restraint is prudent.
Ultimately, we may be able to generate sublime MDMA-like states - at will, to order, and indefinitely - only when the intracellular signal-transduction mechanisms, and regulation of genetic switching beyond the post-synaptic cascade, are better understood. The orchestrated "overexpression" of some genes and the receptor proteins they code, the redesigned "under-expression" of others, and perhaps the selective silencing of gene expression via RNA-mediated interference of anything really nasty, can amplify desirable facets of our consciousness and suppress its darker and more poisonous variants. Thus at one terrible extreme, suicide victims, for instance, tend to show heightened levels of serotonin 5-HT2A receptors. Before death, they show a greater activity in the genetic machinery churning out the 5-HT2A receptor itself. So as well as developing gene-therapy to prevent suicidality - and forestall the whole spectrum of deeply unpleasant para-suicidal and self-destructive states - it should be possible, conversely, to engineer an unimaginably richer love of life, of ourselves and each other by genetically enhancing our own minds. Freedom to optimise (or at least improve) one's genome should prove at least as personally liberating as the freedom to optimise one's drug-regimen. Doubtless a regulatory minefield lies ahead.
One momentous development is perhaps only a decade or so away. In the imminent era of genomic healthcare, we may each enjoy access to a read-out of our own individual genotype i.e. the set of particular forms of genes - alleles - peculiar to each individual who isn't a monozygotic twin [triplet etc]. Harnessed to pharmacogenetics, the study of how an individual's distinctive genetic inheritance affects the body's response to drugs, such intimate genetic self-knowledge should allow the design and prescription of a drug-regimen tailored to each unique person, whether for medical, social, research or "recreational" purposes. At first, only the crudest stratification of patient populations by genotype may be the medical norm. This is because commercial drug companies prefer large markets. Yet eventually we should all have optional access to the gene-expression profile of each neurotransmitter-specific neuronal subtype in the mind-brain. Such access offers scope for fine-grained manipulations of the chemistry of our souls inconceivable in the Dark Ages of pre-genomic medicine.
Genetically personalised medicine offers another bonus. It should eliminate the possibility of idiosyncratic drug reactions caused by genetic abnormalities - for example rare polymorphisms of the cytochrome P450-2D6 system critical to drug metabolism. Owing to genetic polymorphisms in drug-metabolising enzymes, receptors and transporters, a range of drugs beneficial to c.99% of the population can't get regulatory approval. In some cases, valuable licensed medicines are pulled after post-marketing surveillance. This therapeutic opportunity is wasted because, say, 1%, 0.1%, or even 0.01% of people who take such agents suffer severe adverse reactions. The advent of genetically personalised medicine should mean that these atypical cases can be excluded; and given other medication instead. Useful older drugs can be dusted off the shelves and re-licensed. New agents can be developed and given faster regulatory approval.
Psychoactive drug users in particular should benefit from the prospect of genetic self-knowledge. Cytochrome P450 forms a superfamily of hepatic enzymes with hundreds of different isoforms that catalyse the oxidative metabolism of a huge diversity of substrates, including MDMA. The duration of action and/or intensity of the effect of numerous drugs are determined by their rate of metabolism by cytochrome P450. Whereas some "housekeeping" enzymes are expressed constitutively i.e. they are perennially active, other enzymes are expressed essentially only when triggered by the presence of the exogenous chemical. Inducible enzyme isoforms increase both in amount and activity in response to drugs.
The precise role of CYP2D6 in MDMA pharmacology is still unclear. MDMA is not merely a substrate for CYP2D6; it also binds to the enzyme, forming an inhibitory complex. The CYP2D6 enzyme is soon saturated even in efficient metabolisers. Other human cytochromes P450 such as CYP-1A2, CYP-3A4 and CYP-2bB are critically involved in the oxidative metabolism of MDMA. It is possible these other metabolic pathways play an important role in everything from idiosyncratic responses to MDMA to the notorious "loss of magic". If enzyme induction accounts wholly or in part for the loss, then the roots of disenchantment can be investigated and prevented, whether for MDMA or perhaps its still imperfect successors. If, however, central processes of neuroadaptation are at work, either instead or as well, then longitudinal neuroimaging studies comparing the brain-scans of, say, drug-virgins ninety minutes or so after dropping their first magical E (or perhaps its safer successor(s)) with brain-scans taken during their hundredth-odd trip should allow the neurochemical basis of any loss of magic to be pinpointed and reversed. Indeed the magic itself can presumably be amplified, probably more delightfully than an unenchanted Darwinian mind can grasp.
More broadly, genomic medicine will deliver the freedom to choose who or what we want be, both as individuals and collectively as a species. In the long run, a spectrum of mental superhealth that is orders of magnitude richer than anything accessible today can be genetically pre-programmed. "Phenotypic plasticity" (the nearest analogue to free will a molecular geneticist will recognise) can be both vastly extended to enhance personal autonomy and, no less importantly, constrained where it's cruel and unwanted. Thus better designed gene-and-drug combinations can perpetuate truly sublime modes of consciousness whereas, conversely, a predisposition to such ancient Darwinian horrors as sociopathy or suicidality can be genetically cured. A rewritten genome can potentially liberate us from all trace of psychopathy and depression - the enemy from without and, all too often, the enemy within. When taken today, MDMA rapidly banishes the horrors of both. Alas they soon return; the acute effects of MDMA are mostly all too reversible. Prediction is always a hazardous business, but to our descendants, breeding kids with anything like our own corrupt code may seem like wanton child abuse.
Post-Darwinian Medicine
Decoding the human genome offers the promise of lifelong emotional health via somatic or germline therapy. Such well-being may be modulated at will via entactogens-empathogens akin to MDMA; by entheogens, psychedelics, nootropics; or agents from categories currently too exotic to imagine. Or alternatively, our descendants may opt to abandon psychotropic drugs as pollutants of their genetically-enriched minds.The biotechnology revolution throws up darker scenarios too. The spectre of biowarfare, bioterrorism, and perhaps totalitarian state control over our reproductive decisions tends to loom larger in the contemporary imagination than utopian visions of boundless love and joy. Clearly genetic engineering and designer-drugs, like the printing press, can be put to unethical use. Nightmarish dystopias make spine-chilling science-fiction and, maybe more plausibly, better futurology than wide-eyed technophilia. The near future may indeed be bleak. Those of us who aren't morbidly interested in pain and suffering probably underestimate how dreadful primordial Darwinian life can be at its worst. Some mental and physical torment is so bad its victims would snuff out the whole world to end it. Disturbingly, the sense in which its victims could be deluded in evaluating its dreadfulness is unclear.
Yet human nature as encoded in our DNA isn't immutable. Mankind's barbaric track-record to date is an unreliable guide to our post-human future. If Homo sapiens' nastier alleles and their more sinister combinations can be silenced or edited out of the genome, and new improved code-sequences inserted instead, then the pessimists will be confounded. A major discontinuity in the development of intelligent life lies ahead. Providentially, we've learned that the DNA-driven world isn't written in God-given proprietary code it would be hubris to tamper with, but in bug-ridden open source amenable to improvement.
Given our current design-limitations, any planning for a post-human population endowed with invincible mental health sounds ambitious in scope if not messianic in spirit. Even granted that paradise-engineering is technically feasible, the abolitionist project still amounts, by today's lights, to a breathtakingly bold strategic move for our species. It may never happen. Most philosophers assume that suffering will endure as long as life itself. Only the horrific, purposeless cruelty of a living world evolved by natural selection makes an abolitionist agenda of "unnatural" selection so morally urgent. That said, any mental health plan aimed at underwriting lifelong emotional well-being for the world's population no more entails developing a millenarian blueprint for a post-human Utopia, or prophesying the imminent End Of History à la Francis Fukuyama, than the still radically incomplete conquest of "physical" pain dictates specifying the particular kinds of pain-free lives we should all lead. The lifestyle options following success in either case are effectively limitless. Thus any fleshed-out examples of possible post-Darwinian forms of life are purely illustrative. If mental superhealth does become the societal norm, then gradients of genetically predestined bodily and emotional well-being can constitute the presupposed backdrop to the diversity of everyday life, not its focus. Gradients of lifelong happiness can enrich our autobiographical narratives, not supplant them. Ecstasy needn't be orgasmic; though it can be.
As contemplated today, scenarios of a post-Darwinian era of lifelong bliss demand a greater effort of imagination than the possibility of lives spent "merely" without "physical" suffering. The futuristic scenarios feel "unreal". The prospect of lifelong happiness strikes us as far more utopian in conception than the prospect of lifelong bodily health. Yet in both cases, gradients of well-being can play a role informationally analogous to their nastier Darwinian counterparts while shorn of their unpleasant (and sometimes harrowing) subjective textures. Assuming here without argument a functionalist model of computational mind, what's indispensable to intelligence in the broadest sense of the term are the triple processes of information, computation and feedback. The "raw feels" of unpleasantness are neither necessary nor sufficient for intellectual progress. Thus our silicon robots don't suffer anguish, even when we recode their "affect programs"; and it seems they're getting smarter a lot faster than we are.
The existence of lives animated by gradients of well-being should be distinguished from lives spent in a state of uniform well-being. Chronic heavenly bliss, like chronic pain and despair, is a condition that's technically possible to implement in the vertebrate CNS. For good or ill, such uniformity would be a recipe for stasis. The intra-cranially self-stimulating rat or monkey - or human wirehead - isn't going anywhere. By contrast, if a predisposition to gradients of ecstatic well-being is ever genetically encoded as our default mood-spectrum, then critical discernment can be functionally retained, and self-motivation enhanced, without sacrificing the humane ethic of a cruelty-free world. This conjecture isn't idle. Some bioethicists would argue a world without suffering is the precondition for any civilised society. Plausible or not, the lack of any inevitable tradeoff between happiness and critical insight undercuts one ideological obstacle to global mood-enrichment.
Predicting the dial-settings on the emotional thermostats of our descendants is unavoidably speculative. But if (very controversially) post-Darwinian humans will innately feel superwell, albeit in varying degree, precisely what modes might their genetically enhanced and perhaps pharmacologically modulated well-being most plausibly take? Will such well-being be the egoistic happiness of amoral, emotionally self-reliant ubermenschen? Or could being loved-up on Ecstasy, or perhaps long-acting brands of entactogen-empathogen cleaner and safer than MDMA, offer a better model of social life in centuries to come?
This sort of crystal-ball gazing clearly demands scepticism as well as a lively imagination. As Dr Shulgin reminds us elsewhere, any prediction is hopelessly entangled with the wishes of the predictor. Such bias might seem to defeat the enterprise from the start. Fortunately (or otherwise), however, some scientific prophecies can become self-fulfilling if ever their makers acquire the power to implement them. In this instance, technically at least, Homo sapiens will soon have the collective scientific expertise to redesign our own nature. So if we ever aspire to enjoy, say, lifelong ecstasy for our minds and bodies, then we can have it. If we're so minded, then apes can become angels. The ultimate stumbling-block, if there is one, will be traditional Darwinian-era ideologies, not scientific ignorance of how to redesign the molecular machinery of emotion. For sure, the vision of a whole civilisation, and not just an all-weekend rave, founded on the neural substrates of an E-like "Peace, Love, Understanding and Respect" sounds sociologically naïve and (socio-)biologically impossible. Stated so bluntly, the loved-up edition of scientific utopianism is perhaps the most implausible (and unreadable) premise for a sci-fi novel one can imagine. The non-specific prediction of genetically preprogrammed well-being for our descendants is contentious enough as it stands. Any more detailed explorations of the possibility that such enriched well-being might be, say, E-like rather than egoistic are therefore hugely more speculative; and quite probably mistaken. But for the following reasons, a civilization based on relationships of, say, mutual loving empathy and intensified E-like consciousness is not impossible, just far-fetched. At a minimum, it's worth sketching out an extended family of E-like scenarios as a corrective to a routine but unargued assumption that underlies rival predictions. This assumption is that societies based on the behavioural genetics of primate-style dominance-and-submission hierarchies will endure indefinitely. Thankfully, both the reproductive biology and mode of selection pressure at work in the new era of genomic medicine will be different from the evolutionary past. Our genetic programming will no longer be "blind", even if its early (re-)programmers will be only partially sighted. Varieties of (post-)human genotype won't just be quasi-randomly generated via sex, genetic crossing-over and mutation. Instead, genotypes will be purposely (re)designed - even if the early designers barely know the ramifications of what they'll be doing.
For we're presently on the brink of the era of "unnatural" selection. Throughout the living world, a regime of blind natural selection acting on effectively random mutations has governed the evolution of information-bearing self-replicators since the origin of life itself. The Darwinian Era has lasted for over four billion years. Most recently, in the aftermath of the post-Cambrian explosion of multicellular animal life and the evolution of central nervous systems, selection pressure has created suffering beyond belief. Mercifully, a regime change is imminent. Within a few centuries at most, intelligent life will be able to rewrite the vertebrate genome and redesign the planetary ecosystem. Sooner still, if we want our genetically enriched (grand-)children to be happy, then in the impending reproductive era of preplanned designer babies we will also have to choose - either actively or by default - whether the kinds of heritable well-being our offspring enjoy will tend to be solipsistic or social, orgasmic or intellectual, hypomanic or serene, loving or self-centred, or perhaps ultimately take forms that can't be grasped by the contemporary Darwinian mind. Whatever criteria are used, an increasing range of genotypes will soon be chosen in deliberate anticipation of their phenotypical effects. Needless to say, no such calculated sets of genetic decisions can be taken by prospective breeding couples at present. "Genetic choice" in the early 21st century usually means nothing more ambitious than choosing the gender of one's child, often in less than ideal circumstances. Yet as the human genome is deciphered, and eventually the "transcriptome" and proteome beyond, a staggering extension of freedom of choice will be thrust upon us. As wiring up the neural reward centres with microelectrodes shows, the practicalities of inducing - and then sustaining - the neural substrates of bliss are technically quite easy. Viewed as an engineering problem, no one needs to suffer; suffering is an unnecessary evil. This is true even with today's embarrassingly clumsy interventions. Modulating bliss in controllable ways is trickier - whether by drugs, microelectrodes or gene-therapy. Short-term technical snags aside, our state-space of life-enriching options is poised rapidly to expand. Of course even with utopian biotechnology and mature nanotechnology, constraints won't be absent. The menu of practical choices on offer to our enriched descendants isn't entirely limitless. For instance, decades spent in unceasing, paroxysmal super-orgasms, or perhaps immersion in fantasy wish-fulfilment in virtual reality software, may well be viable lifestyle options one day for individuals. They are technically feasible to implement. Yet it's hard to imagine parents wanting such modes of existence for their kids, or to devise evolutionary game-theoretic models where the prevalence of genotypes that permit such lifestyles could be globally stable. In any post-Darwinian reproductive era ahead, "unnatural" selection pressure will still be at work, at least until the abolition of senescence brings the throwaway era of traditional DNA mortals to a close. Thus any currently foreseeable civilisation will be social in character, not quasi-solipsistic. If so, then one urgent challenge will be to make our social interactions less emotionally costly. Today, for a few hours, MDMA offers perhaps the richest chemical tool for social intimacy in existence. If nothing else, the MDMA experience demolishes the conventional wisdom that "artificially"-induced happiness must be amoral and selfish - hedonistic, one-dimensional and shallow. More generally, the sense of heightened authenticity, love and self-insight induced by entactogen-empathogens will no longer seem an escapist holiday from Real Life when entactogenic-empathogenic states can be sustained indefinitely - whether by gene-therapies or soul-medicines or varieties of both. In theory, at least, mental superhealth can become the new benchmark of consensus-reality against which any departures are defined, not drug-induced psychotic episodes.
It's safe to say MDMA itself isn't going to change the world. Yet as a taster of what's feasible in post-prohibitionist culture and a possible genetically-enriched future beyond, the MDMA experience shows social life at its best. MDMA promotes a more altruistic mode of consciousness than has maybe ever existed on the planet, certainly among testosterone-driven young males. On MDMA, one can love thy neighbour as thyself; and the lion can lie down with the lamb. Feelings of hostility, bigotry and intolerance evaporate. Competitiveness is replaced by love, tolerance and respect. Such social harmony seems "unnatural" if not miraculous when viewed from the poisonous miasma of mainstream society. For outside the embraces of loved-up ecstatics, we tend to pay a terrible price for the benefits of group living. The costs of social existence are attested by the grisly chronicles of human history, Gibbon's "register of the crimes, follies, and misfortunes of mankind". It's not as though we have much choice about living together. Even in the absence of MDMA, human beings are still compulsively sociable. This compulsion to socialise is generally seen as healthy rather than dangerously addictive, despite the traumas it brings. In abnormal conditions of social isolation, the personality starts to deteriorate: solitary isolation, whether real or figurative, is rightly viewed as a cruel and unusual punishment. Our genes, via the reward pathways and the neural projections they code, make us chronically hooked on the company of others. Our very identities are bound up with our social roles. We are physiologically dependent on the opioidergic, oxytocinergic, dopaminergic and serotonergic mechanisms that friends, family and colleagues may trigger within us.
At its best, this chronic dependence on human company gives short-term highs and even warm afterglows. But dependency, craving, and withdrawal-reactions are common when significant others are taken away from us or adulterated. Purity varies; supply is erratic; and adverse reactions are common. Cue-elicited craving readily triggers relapse. Our sociability can spin out of control into a financially ruinous habit. We spend lots of time and effort thinking about how to get more of whoever stimulates our mesolimbic reward circuitry most vigorously, possibly romanticised under more poetic descriptions. Without friends, lovers or family, most people tend to become lonely, sometimes agonisingly so. In extreme cases, loneliness and lovesickness can induce suicidal despair. Bereavement and abandonment can be as traumatic as heroin-withdrawal; and they share similar neural substrates.
Alas given a Darwinian genome the compulsion to socialise is all too often a dangerous, self-destructive addiction. Living without Ecstasy, we are predisposed by our genes to conflict with each other: mothers and children, men and women, brothers and sisters, friends and relatives - all have different and often conflicting genetic interests. Only monozygotic ("identical") twins may hope to be spared this insidious rivalry. Camouflaged genetic conflict underlies our disposition to squabble and fight amongst ourselves, even though we compulsively need each other's company too. Thus testosterone-driven males find themselves enacting decades-long competitive rituals to attract and retain genetically superior mates. Competitive status-seeking in its subtle - and not-so-subtle - guises is a cross-cultural universal, deeply rooted in our biology. Conflict to the point of warfare is genetically predisposed in our make-up. It's endemic to human society. Right from conception and the implantation of the conceptus in the womb, genetically-driven conflict plays itself out, often leading to trauma (e.g. preeclampsia etc.) for mother and unborn child alike. Later on, even the noblest of human sentiments are fragile. Love all too easily turns into hatred, admiration into contempt. By way of illustration, consider, say, proverbial "lover's quarrels". There are innumerable "proximate" explanations of why star-crossed lovers often argue so painfully and vehemently. But the "ultimate" evolutionary explanation seems to be, at least in part, that tempestuous rowing and its consequences serve as a brutal but effective way for prospective breeding couples to test each other out. Evolutionary psychology suggests that, from a gene's eye view, it's better to discover if a prospective partner will let you down sooner, when (s)he's goaded under conditions of stress, rather than later after you've sunk a substantial investment of time and resources in the partnership. Arguing can be traumatic; but the capacity to do so is [genetically] adaptive.
Human relationships can bring psychological rewards too. Anyone having fun right now would find this drumbeat of misery, heartache and emotional squalor all a bit overblown. Yet if anything the nastiness alluded to here is understated; the worst pains in life are inexpressible. Under the genetic status quo, most of us are condemned at different stages of a lifetime to re-enact the messy - and sometimes desperately sad - personal dramas of our ancestral past. TV soap-operas and teledramas serve to sanitise just how emotionally unpleasant Darwinian life can be. For as human generation succeeds human generation, we replay the age-old sagas of sexual betrayal, jealousy, loneliness, and rejection. We are forced to endure the savage competition of lookism - an inadequate, frivolous term for a cruel and omnipresent phenomenon in human society. Less colourfully, a multitude of pettier but still wounding frustrations, humiliations and misunderstandings can mar daily social life and sour personal relationships.
The genetic rot goes deeper. Evolutionary psychiatry suggests that the cross-culturally ubiquitous phenomenon of depression, and the wider spectrum of depressive and dysthymic disorders, is not a genetically dysfunctional anomaly. Counter-intuitively, a conditionally activated tendency to depression may represent a fitness-enhancing adaptation to group living. The (involuntary) capacity for depression is one of a number of ancient, genetically adaptive mechanisms and strategies for dealing with "social defeat" in a tribal environment. Group living conferred advantages on otherwise vulnerable individual primates on the African savannah. Embracing tribal life forms a valuable defence for a puny "naked ape" against big predators. But thanks to the pressure of sexual selection, human tribal society imposes a cruel pecking-order of subordination relationships among members of the tribe. For sure, depression has many proximate causes; there are many subtypes of depression; and not all depressive moods and behaviours are genetically adaptive. Yet viewed in evolutionary perspective, a syndrome of sustained melancholy, behavioural suppression, and a preoccupation with personal failure and inadequacy is the internalised correlate of the yielding or "losing" behavioural sub-routine. On this "rank theory" hypothesis, the involuntary activation of submissive and depressive states is an unpleasant but effective defence-mechanism for weaker individuals where any tendency to initiate or escalate conflict with a powerful dominant rival might easily be disastrous. Thus states of depression or low mood ensure the weak "keep their heads down" and don't overreach themselves. By contrast, the (hypo-)manic spectrum of mood and behaviour is a manifestation of the "winning subroutine". Clearly, not all submissive people are unhappy, and not all dominant and/or aggressive people are (hypo-)manic. So there are complications to the hypothesis and a legion of exceptions. A capacity to switch mode can sometimes be adaptive too; hence the probable evolutionary origins of bipolarity. Yet down at the bottom of the social heap, there are proportionately far more crushed and wounded spirits than there are at the top. Socially dominant Alpha males tend to be temperamentally expansive and optimistic. Conversely, even life's "winners", if deposed and defeated, may become depressed themselves, and slink away to die, metaphorically or otherwise. Depression in human societies is far more prevalent than (hypo-)mania, albeit far less visible. This greater prevalence probably reflects conditions in the evolutionary environment of adaptation where the spectrum of depression, mania and bipolarity first arose. But whatever the ultimate evolutionary roots of mood variation and affective disorders as interpreted by rank theorists, contemporary humans in all known societies are obsessively status-conscious. Status-competition corrupts personal relationships in societies stratified by caste, class and money alike. Even apparent counter-examples don't challenge the generalisation. Thus societies based around the potlatch, the Pacific Coast Native American custom of conspicuous gift-giving rather than wealth-accumulation, reflect a disguised expression of competitive power relationships. Indeed the tradition of rival displays of gift-giving finds echoes today in the competition between billionaire American plutocrats to endow the biggest charitable trust foundations.
The poison of competitive status-seeking might seem incurable. ["If everybody is somebody, then nobody is anybody"; "It's not enough to succeed. Others must fail"] Yet short-term symptomatic relief for this syndrome already exists; and its long-acting analogues may one day offer complete remission. Taken communally, MDMA induces an almost miraculous transformation in the structure and relationships of any social group. At MDMA-animated raves, no one who's loved-up is trying to "diss" anyone else. MDMA abolishes the desire to put anyone down. On MDMA, primate dominance hierarchies dissolve in an egalitarian love-in. A lifetime's inferiority-feelings, snobberies, and status-anxieties dissipate in a flood of augmented serotonin and dopamine release. Intriguingly, the euphoria experienced by MDMA users doesn't take the form of uncontrolled manic excitement, even where the drug induces "behavioural activation". MDMA's indirect, serotonin-mediated enhanced dopamine-release produces psychostimulant, emotional and perceptual effects that feel very different from crude dopamine-releasing amphetamine. Even the most animated ravers taking pure MDMA tend to experience a profound sense of inner calm, a "peace that passeth all understanding". Identifying the neurochemical signature of states combining inward serenity and outer dynamism presents a wonderful therapeutic opportunity. The contrast between raves packed with loved-up clubbers on hugdrugs and parties fuelled by alcohol or cocaine is striking.
Back in the harsh, E-less world, inferior social status is associated with low serotonin function and low mood. Thus dominant males tend to have far higher serotonin function, as measured by CSF 5-HIAA levels, than subordinate males. The neurological basis of social rank order can be investigated by various manipulations. Experimentally boosting or depleting the serotonin levels of social animals enhances or sabotages an individual's place in the pecking-order. Revealingly, there are also gender differences in serotonin activity. The mean rate of serotonin synthesis in men is over 50% higher than the mean rate of serotonin synthesis in women. Women are more sensitive than men to both the MDMA magic and MDMA's adverse side-effects. Women are also more likely to suffer from the post-E serotonin dip; more prone to depression; and more likely to benefit from Prozac. Yet such comparisons are invidious. Men and women alike of any social status at all can flourish far better in E-like states - while they last. Unfortunately, communal E-like consciousness simply isn't sustainable via chronic MDMA use. In wider E-less society, "winners" probably don't do [serotonin-depleting] drugs, though idealistic E-users might suggest that zero-sum status-games are best not played at all. For better or worse, a heavy, serotonin-depleting E-regimen can disrupt the user's social status in the competitive urban jungle - and probably elsewhere. Admittedly, there are too many confounding variables to test this hypothesis in methodologically rigorous studies on humans. Even so, a proposed "E-users are losers" research proposal is more likely to gain official funding than a well-controlled trial of, say, the health benefits of MDMA-assisted psychotherapy.
Of course the aspiration for a civilisation founded on relationships of shared love and respect sounds impossibly idealistic. A society based on "winners" and "losers" intuitively strikes us as natural. With today's genes and the kinds of culture they promote, adversarial social relationships are probably inevitable. Like depression, the evolutionary roots of everyday sociopathy run deep. For speculatively, applying here Richard Dawkins' "extended phenotype" theory, not merely has it been genetically adaptive for weaker social primates to have an inbuilt conditionally-activated capacity for depression, it can also be genetically adaptive for Alpha males (and aspiring Alpha males) to subdue potential rivals by making them depressed too. Perennially chastened, socially anxious and chronically depressive potential competitors are less likely to be sexually active and promiscuous. Crushed, anhedonic and submissive, they are less of a challenge to the inclusive fitness of one's genes. Happy, dominant, extroverted males, by contrast, are potential sexual rivals who directly or indirectly threaten one's reproductive success. Thus we witness their downfall with equanimity. Even within the bounds of holy wedlock, too much happiness for one's nearest and dearest doesn't always suit one's genetic interests. A cowed and depressive wife, whose only solace in life is looking after the kids, can be less threatening to one's genetic prospects than an exuberant, sociable and possibly sexually adventurous bundle of joy. If we are looking for an evolutionary perspective on why we often behave so vilely to each other - sometimes seemingly gratuitously so - then this kind of sexual selection pressure offers one possible explanatory framework. If it is adaptive to have others exhibit a spectrum of behaviour characteristic of low mood or high social anxiety, then other things being equal, alleles and allelic combinations may flourish if they conditionally promote the capacity to induce such anxiety and depression whether in strangers or tribe members who aren't allies or close kin - and if occasion demands, even in those who are both. Depending on a lot of other factors too, the (behavioural effects of the) happiness of others, male or female alike, can indirectly detract from our own Darwinian fitness. Consequently their perceived happiness doesn't tend to give us as much joy as moralists might wish. Sad to say, reports of good fortune befalling our fellows do not always inspire a warm glow of vicarious satisfaction. On the contrary, news of another person's lottery-win, for instance, or its traditional counterpart on the African savannah, is liable to trigger involuntary feelings of jealousy and resentment, or at best an envious ambivalence. Of course, it's worth stressing that natural/sexual selection doesn't care about the subjective textures of misery or happiness per se. Selection pressure works on the spectrum of behaviour such mood traits engender. What was selected for [as distinct from adventitiously selected] in the ancestral environment of adaptation wasn't the capacity to make others feel miserable as distinct from behave miserably. To selfish DNA, our suffering itself is incidental. The distinction, however, is of limited comfort to its victims.
Fortunately we're not systematically spiteful, even though we're not naturally loved-up. If human malice were really genetically hardwired, then any nostrums for social reform, life-enriching lovedrugs or improving the vertebrate genome would be futile. Thankfully, a malignant streak of human nastiness is matched by a common if ineffectual desire to improve ourselves and help others. This good-will just needs genetic and pharmacological amplification.
So granting human beings no more than a minimal and diffuse benevolence, what can be done to make us temperamentally nicer to each other as well as happier and smarter? Would we individually and collectively be better off if perpetually loved-up on more advanced and sustainable analogues of E? Or are loved-up ecstatics just too vulnerable to genetic invasion by "defectors" and wolves in sheep's clothing for such genes or allelic combinations to flourish? Vulnerability to predatory and Machiavellian genetic rivals is presumably the reason why sweetheart suckers living in blissfully E-like states are thin on the ground in the drug-free Darwinian world. What reasons are there, if any, for predicting that the nature of adaptive traits in the era of genetic engineering will change in ways that make beautiful minds more widespread?
If genetic engineering or rational drug design are to deliver us from the Darwinian rat-race into everyday states of ecstatic grace, or anything at all like it, then there are short-term and wider evolutionary constraints to be overcome. Truly far-sighted genetic re-programming is a formidable challenge. Some genetic manipulations may involve computing the interactions between dozens or ultimately hundreds of alleles. Often their contributions to mental and behavioural traits won't be additive but dependent on a plethora of environmental contingencies. This Problem of Conditional Activation threatens a combinatorial explosion of possibilities to calculate. It presents a daunting task of prediction and control. Other interventions, however, might seem (comparatively) more straightforward. For instance, the action of testosterone, and its hormonally active dihydrotestosterone metabolite, is in large part responsible for war, social violence and competitive dominance behaviour, territoriality, sexual aggression, reduced male life-expectancy, and going bald. The genetic and/or pharmacological manipulation of testosterone may play a vital role in undercutting the darker horror scenarios for the future so popular in the science-fictional literature.
Yet first there are many problems to be resolved here too. Testosterone can't, realistically, just be edited out of the genome, as distinct from edited and re-regulated. The eradication of testosterone would indeed spell a world without war. But androgenic hormones can't be deleted altogether, even if the option of rearing functionally emasculated or chemically castrated offspring were an idea palatable to prospective parents, an unlikely prospect right now. Testosterone is the stereotypical "male hormone". Yet testosterone is present in women too, albeit in smaller amounts: it's important to female sexual response, just as it's responsible for spontaneous nocturnal erections in males. Testosterone plays a role in female bone-strength, muscle-mass and a general sense of well-being. Moreover, expository convenience aside, androgenic hormones are no more intrinsically evil than the MDMA molecule is intrinsically good. Thus testosterone promotes what might be described as "strong-mindedness". Today the trait of strong-mindedness fosters what's often little more than callousness in pursuit of unworthy ends. But not always. Even in a mature post-Darwinian civilisation, most of us may well prefer to cultivate "strong personalities". The popularity of performance-enhancing anabolic steroids with athletes and bodybuilders stems only in part from the way such drugs enhance strength, power, speed, endurance and muscle-mass. For anabolic steroids are popular because they can also act as mood-elevating, mind-toughening personality-pills. Taking anabolic steroids induces a sense of well-being sometimes amounting to euphoria, an increased tolerance of stress, and a sense of competitive "edge". The price of using such drugs can be hypermasculine aggressiveness ["roid rage"], increased dominance behaviour and even a propensity to sexual violence. Normal endogenous male production of their native anabolic counterparts is risky enough already. If our species is to survive its newfound capacity to build weapons of mass-destruction, and tackle the genetic origins of male violence and all-round nastiness, then we must somehow curb the biological roots of masculine aggression. This particular intervention strikes us as a disconcerting prospect. Darwinian sexual and gender identities are central to social existence today, and usually integral to who we think we are. However, the long-term role of the Y chromosome in the evolution of intelligent life is uncertain; and the ethical value of testosterone-driven masculinity is unproven at best. The phenomenon of sexual reproduction itself has only persisted and evolved as a defence against parasitism; an additional mechanism that promotes genetic variability is a powerful weapon in the evolutionary arms race against pathogens. In the new reproductive era ahead, however, genetic diversity can be intelligently pre-planned. So at the very least, enlightened biomedicine should be able to edit out a predisposition to the more sociopathic forms of masculinity from the genome. More far-reaching strategies can be contemplated too. On the other hand, recalling H.G. Wells' The Time Machine (1898), we don't want to turn into enfeebled and weak-minded Eloi, even if we live in a world without Moorlocks. It's good to wake up each morning feeling ready to take on the world and win, even if we eventually discover that the rest of the world is on our side; and some day it may be conspiring to help us.
Today the world generally isn't on our side. Low testosterone function is associated with social defeat, passivity and subordination. Low testosterone levels are also implicated in depressed mood. The syndrome of depression has both proximate and evolutionary roots. Depression is popularly viewed as a sign of weakness; and folk-wisdom is right. Such a perception leads to its systematic underreporting, especially among males, thereby painting a falsely rosy picture of (male) mental health. Depressive illness is reported to be twice as common among women as men. Conversely, it's sexy for men to be cool, confident and 'sussed' - the sort of personality often faked if you aren't, though for evolutionary reasons depressives find it harder to bluff. Therapists and sensitive physicians may take determined steps to reassure their clients that depression isn't a sign of weakness. Alas this assurance typically isn't true. Depressives characteristically tire quickly, act ineffectually and give up too easily. Potential new antidepressants are correspondingly tested for their capacity to reverse the learned helplessness and behavioural despair induced by chronically "stressing" [torturing] non-humans in "animal models". Whereas exuberant hypomania is a signal of strength and resolve, albeit a risky signal, depressives can't pursue their projects with fanaticism, nor can they work indomitably to pursue what they believe to be morally right. For their capacity to anticipate reward is blunted. Life for depressed people too easily seems meaningless, absurd and pointless - the nihilistic polar opposite to a hyperdopaminergic sense of urgency and significance. For the mesocorticolimbic dopamine system mediates not just the salience and intensity of anticipated reward; it also determines strength of will. By contrast, the spirit of depressives is easily broken; and there's no natural remedy for weakness of will.
This grim diagnosis isn't a counsel of despair. On the contrary: well-designed genetic and pharmacological interventions should in principle allow weaker spirits to be invigorated and frailer personalities empowered. With better drugs and better genes, one's idealised persona can be made flesh. We'll soon have the option of making ourselves stronger, better-motivated and more steely-minded in character than even the bravest palaeo-Darwinian primitive or Nietzschean ubermensch. It's an open question whether such strength of character will be egoistic or empathetic, cocaine-like or E-like, or something different altogether. Yet with the right gene-and-drug combos, we can be superheroes, even if the need for heroism may shortly pass. In the meantime, innovative pharmacotherapy and/or genetic medicine will be vital if the weak-mindedness and weak willpower blighting so many lives today is to be overcome.
Weak-mindedness takes many forms. One effect of administering MDMA is the way it eliminates jealousy. Even anti-abolitionists, normally so eager to hymn the character-building virtues of suffering stoically borne, rarely find many positive words to say about the ennobling attributes of the green-eyed monster. Jealousy is a persistently nasty, vicious, and pervasive feature of Darwinian human social relationships. It's also about as voluntary as sneezing; and far harder to cure. Like MDMA, SSRIs tend to diminish jealousy. SSRIs also act more sustainably than short-acting clubdrugs. But SSRIs also tend to diminish the intensity of being in love. On MDMA, by contrast, people of either sex can and frequently do spontaneously embrace and caress each other - complete strangers as well as intimate friends. On MDMA, everyone naturally tends to love each other, almost as if we were clones rather than genetic rivals.
Such behavioural effects present a bizarre and perhaps disturbing spectacle to the E-less Darwinian outsider. Yet the lessons to be drawn from the use of today's crude hugdrugs and lovedrugs extend far wider than the recipe for a good weekend out clubbing. One way to put the world to rights invokes the tired nostrums of socio-economic and political reform. Such social engineering hasn't proven effective at curbing the frightfulness of life to date. Pursued in a biological vacuum, so to speak, any kind of environmental approach to building a world without cruelty, fear and pain is bound to fail. The other, biologically based strategy for saving the world will involve treating our, say, congenital androgenic, serotonergic, opioidergic, dopaminergic, PEA and endocannabinoid dysfunction via gene-therapy and rationally designed pharmaceuticals. Critically, it entails choosing kinder genotypes for our offspring. This option doesn't amount to a very soul-stirring prospect. Like our notions of psychoactive drugs, the concept of "eugenics" is horribly tainted. The word itself, originally a coinage of Sir Francis Galton (1822-1911), is indelibly tarred with the pseudoscientific quackery of the Third Reich - though it's worth recalling that Nazi "race-hygienists" didn't use happiness as their touchstone of genetic excellence. Yet the lethal dangers posed by the genetic status quo coupled with advanced military technology are far greater than the risks of a genetic reform program predicated on the goal of world-wide personal happiness. Warnings from history aside, unless the Darwinian masculine identities of our evolutionary past are superseded, then jealousy, conflict and warfare will go on for ever (or kill us off); and the prophets of doom will be right.
Alas Ecstasy itself is something of a false prophet. MDMA-induced love is no more everlasting than its older and fitness-enhancing counterpart. Two days after taking the magic lovepill(s), the drug-catalysed outpouring of affection has subsided. "Natural" love sometimes lasts longer; but Darwinian love is still ephemeral, eventually killed off by receptor desensitisation and down-regulation no less effectively than E-induced love is ended by serotonin depletion. For the fickleness of Darwinian affection has hitherto been genetically adaptive. It's an adaptation that remains a shabby substitute for genetically-underwritten true love. Only by subverting some exceedingly cruel feedback-inhibition mechanisms can the depth and range of our affection for each other be enriched and sustained. As it is, most Darwinian social life is soulless and loveless. But our genes do allow their vehicles to fall in and out of love with a small percentage of prospective mates in ways that tend to serve our reproductive success. In a largely anonymous mass-society, love and affection are in even shorter supply than among tribal hominids in African prehistory. Where love does sporadically flicker or flare up among us, its expression is tightly regulated. E-less love is rarely all-embracing: such Darwinian love tends to be jealous, possessive and exclusive. The law and social sanction impose penalties for loving too much or too little, loving the wrong person at the wrong age or the wrong gender. "He who is rational about love is incapable of it"; but this isn't true in the eyes of the law or of our peers.
At MDMA-driven raves, by contrast, women can feel safe in public, gay people feel truly at ease, and sexually straight or bisexual clubbers can express love and affection for each other free from overt or internalised homophobia. Taboos on touching and the whole gamut of tactile experience are relaxed. The body no longer feels like a prison for the soul but an extension of it. The classic dopaminergic psychostimulants like cocaine promote a hard-edged, don't-touch-me egoism. MDMA promotes intimacy, warmth, and an empathetic sense of other humans beings as fellow subjects rather than objects.
Of course, after a weekend of being "loved-up", mood-congruent post-E "reality" soon sets in. Did one really let slip those gushing effusions to strangers one barely knew? Did one really hug that hateful brute of a rival for the affections of one's heart's desire? Viewed from [state-dependent] "reality" again a few days later, being nice to everyone, truly loving oneself, and feeling (and being) wonderful all seem faintly embarrassing, perhaps even a chemically-fuelled madness. "It was the just the E talking". One may recall from English literature the effect of taking soma, the "ideal pleasure drug" featured in Aldous Huxley's uncannily prescient Brave New World (1932). After John the Savage threatens to disrupt their soma supply, the angry low-caste Deltas riot. They are promptly pacified by the riot police with soma-gas, and the rioters end up hugging each other:
"Two minutes later the Voice and the soma vapour had produced their effect. In tears, the Deltas were kissing and hugging one another - half a dozen twins at a time in a comprehensive embrace. Even Helmholtz and the Savage were almost crying. A fresh supply of pill-boxes was brought in from the Bursary; a new distribution was hastily made and, to the sound of the Voice's richly affectionate, baritone valedictions, the twins dispersed, blubbering as though their hearts would break. "Good-bye, my dearest, dearest friends, Ford keep you! Good-bye, my dearest, dearest friends, Ford keep you. Good-bye my dearest, dearest..."Too far-fetched? In 1998, a former South African government scientist told a hearing of the Truth Commission that the minority Apartheid government had planned to use MDMA on rioters. Desperate to retain their faltering grip on power, the embattled regime apparently ordered its chemists to make one tonne of Ecstasy for riot-control. Thus the Calgary Herald (10 June 1998) reports:"Dr. John Koekemoer, former head of chemical and biological weapons research, at the secret Delta G facility, said he disapproved, "I did not believe Ecstasy was a good incapacitant and I told my superiors that", he told the commission, which is investigating human rights abuses during the apartheid era. "Ecstasy enhances interpersonal relationships. I told them I did not want to kiss my enemy."The scary notion of kissing one's enemies, perhaps half-recalled cameos from Huxley's satirical fiction - and the reality of strangers of either sex at raves hugging each other on E and telling each other how wonderful they are - contribute to the perception that E-like states of blissful empathy are inauthentic, shallow or false. How can the MDMA experience have true emotional depth if the cosmic hug-bunny of the dance-floor reverts back at the office next week to his old Darwinian mindset - and the (anti-)social vices it spawns? A corrosive cynicism easily sets in. For that's the nature of social reality, an emotionally frazzled post-Ecstatic may reflect, not the magical interlude of Peace, Love and Understanding and Respect.Sadly, in today's world, this may be so. The depressive realism of the serotonin-depleted and jaded cynicism of the chronically world-weary are often justified. Yet our descendants may recognize that we are the sociopathic emotional primitives in the grip of an affective psychosis. Jealousy, envy, resentment, ridicule, hate, anger, disgust, spite, contempt, schadenfreude and a whole gamut of nameless but mean-spirited states we undergo each day are a toxic legacy of our Darwinian past. More commonly, perhaps, our genetic make-up ensures we simply feel indifference to the plight of all but a handful of significant others in our lives. Right now, for instance, one knows dimly at some level that there is frightful and preventable suffering in the world. Yet most of us feel no overpowering moral urgency to do anything about it. Idealists might vaguely entertain the second-order desire to care more deeply and give, say, a larger proportion of one's money to Third World charities dedicated to those who need the resources more urgently than we do. Yet the biological roots to sustain "saintly" self-sacrifice just aren't there in most of us. In contrast, taking MDMA can give rise to a prodigious sense of compassion in even the otherwise morally inert. Regrettably, such compassion is usually ineffectual; it's too short-lived to do much good. If and when we understand the neurochemical basis of empathy, however, then sustaining the molecular substrates of empathetic love can turn boundless compassion into an automatic reaction to distress, not a sign of drug-induced psychiatric disorder. Intervention can go further. If we decode and opt to amplify the molecular machinery of volition too, then such heightened compassion can be translated into effective action.
Fortunately, compassion if not empathy for others may ultimately be redundant. In the long run, if biotechnology can be used to eradicate suffering from the living world, then a shared celebration of life, not sympathy for the misfortunes of others, may come to seem as natural as breathing. Yet right now too many people walk the Earth who have no cause to celebrate anything. Therapeutic agents designed to deepen empathy and sustainably awaken our compassion are a priority. The functional prototype of what's needed exists today in the form of a fast-acting hugdrug; but MDMA itself is not the recipe for perpetual sainthood.
The design of richer functional analogues of MDMA entails more than finding medicines to make us sweeter-natured. Improving human nature is perhaps ethically all-important, but MDMA is also an entactogen - a more elusive concept than that of an empathogen. MDMA offers "insight without fear" (Dr Shulgin). The nature of entactogenesis is far harder to fathom, let alone communicate, than the nature of empathy. The word for such states comes close to being a primitive term, its sense semantically inaccessible to the MDMA-naïve. The clarity of MDMA-mediated self-insight is perhaps a form of what Dr Charles Tart calls "state-specific knowledge". E-less cynics may be sceptical. Just what's the propositional content of this so-called "insight"? Couldn't it be delusive? ["It's not hard to hear voices. It's knowing whether they tell you the truth."] But on pure MDMA, the subject can inwardly access the kind of person s/he wants to be; "the ideal me". Whether this idealised self-identity is created or discovered may be philosophically debatable. But the deeply-felt sense of authenticity and emotional self-honesty of the MDMA experience is an unexpected delight. One just won't ever get to read about its nature in the peer-reviewed Journal of Introspective Studies.
In Western culture, a capacity for reflective self-insight is not highly prized. Introspective genius and a talent for meditation aren't respected in either academia or business. Nothing in our education system is geared toward making young people feel that introspective self-analysis, enhanced self-awareness or personal growth matters in the slightest. How can they be tested, graded and quantified? What's their market value? Anyone in Western society with a tendency to quiet contemplation is likely to be stigmatised as lazy, feckless and unenterprising - unlike the sound and fury of the lionised Man Of Action, and his larger-than-life ego on whose life-energies lesser mortals may feed. In similar manner, our (limited) vision of future civilisations tends to focus on their technological marvels - and the supposed Darwinian dominance-battles of their science-fictional inhabitants - rather than on odysseys into the inner depths of their souls. Yet the design of long-acting entactogens - and their neurological analogues - should allow introspective depth and a capacity for higher-order self-reflection to be fabulously enriched as well. Tomorrow's counterparts of today's bunch of furtive adolescent introspectionists won't have to shuffle around faint little tickles of thought. Drugs to enrich self-insight and heighten self-reflection may eventually become commonplace. They may be distributed as freely as aspirin if not smarties; and prove safer in excess than either.
This prospect is some way off. Full-blooded pharmacological and genetic emancipation is still decades away. Even so, we are poised to acquire a literally life-transforming technology - a toolkit for enlightenment powerful enough to implement Heaven-On-Earth and beyond - yet we balk at the sorts of public health policy decision needed to accelerate the transition. An enriched conception of mental health is blocked by entrenched elites who've never sampled what they outlaw - whether designer genes or utopian pharmacology. In the jaundiced eyes of (most of) the older generation, Ecstasy and rave-culture are an aberration, not a portent. "Peace, Love, Understanding and Respect" sounds like a hollow slogan. Today, in the wider world, the words can't be anything else. Ecstasy itself is too short-acting, unsustainable and neurotoxic at high doses to form part of anyone's global health plan. But a permanent distillation of the MDMA magic, if feasible, offers an extraordinary if unorthodox vision of one post-Darwinian paradise to come.
Beyond MDMA : mental superhealth?
Moore's Law in computing is named after semiconductor engineer and Intel co-founder Gordon Moore. It states that processing power in computers doubles every eighteen months or so. Moore's Law has roughly held good since 1965 when it was first propounded. It's a rule-of-thumb about how many transistors we can cram onto successive generations of chip rather than a fundamental truth about Nature. Yet the trend it captures seems set to continue, at least until chip designers run up against the physical constraints of the nanoscale later next decade, or perhaps until quantum computers allow calculations orders of magnitude more powerful than today's toys.Unfortunately, the dizzying rate of technical progress that Moore's law quantifies hasn't been matched by an analogous law of progress for generations of human mental health. On average, we are probably no happier than our malaise-ridden hominid ancestors. We aren't noticeably fonder of each other. By way of consolation, we can take refuge in the pre-scientific notion that happiness is unquantifiable. Yet if such quasi-objective indices of mental health as suicide rates are anything to go by, then we would probably be psychologically better off as hunter-gatherers. Over 800,000 people in the world took their own lives last year. The World Health Organisation (WHO) estimates that this figure will rise to around 1.5 million by the year 2020. Here in the UK, suicide is the most common cause of death for men under 35 years old. Globally, several hundred million people are clinically or "sub-clinically" depressed; and a spectrum of chronic anxiety disorders afflicts further hundreds of millions more. Even as we progressively conquer physical disease as conventionally defined, the toll of psychological distress is still rising. Admittedly, "mental illness" and "mental health" are value-laden, ideologically contested terms. Even the new scientific discipline of biological psychiatry is inescapably culture-bound. Yet "Progress" that doesn't leave us emotionally better off would seem something of a misnomer.
Not merely has there been no discernible growth in average mental health to match the tempo of scientific advance, technophobes claim there never will be such a mental health revolution. As long as we rely on the same legacy wetware to animate our lives, the neo-Luddites and religious fundamentalists may even be right. Our levels of well-being - and ill-being - compute fitness functions that served the inclusive fitness of our DNA in our ancestral environment in Africa. Our genes didn't design us with the emotional welfare of their throwaway vehicles in mind. So the genetically adaptive hedonic treadmill - for many of us better named the dolorous treadmill - ensures that average levels of well-being/ill-being of Darwinian life remain stagnant. Six months after winning the national lottery or becoming quadriplegic in a catastrophic accident, the winner/victim statistically reverts to his or her average level of ill-being/well-being before the win/trauma. Hence such affirmations as Rajinder Johar's "The Joy of Quadriplegia". Illustrating the hedonic treadmill at its most extreme, "locked-in syndrome" leaves its victims paralysed. The subject is fully conscious but unable to move any extremities, talk, or make horizontal eye movements. Yet in the words of James Hall, longest surviving (2002) American victim of a midbrain pontine stroke: "In some ways, my stroke was a blessing....Since my stroke, I've published books, articles, poems. I'm busier and happier than I've ever been." Completely paralysed, Mr Hall communicates by focusing on particular letters that his computer picks up from his limited eye-motions.
Such triumph-of-the-human-spirit stories are comforting, up to a point. The downside of the emotional homeostasis they reflect is that millions of temperamentally depressive and dysthymic people would feel gloomy in the Garden of Eden. Again, this hypothesis isn't easy to test rigorously. The more dramatic manifestations of emotional homeostasis at work are hard to investigate ethically in well-controlled prospective studies. Anecdotes and impressions aren't science. Yet the cumulative evidence for a genetically constrained "set-point" in our pleasure-pain axis is compelling. The dismally low dial-setting doesn't bode well for any utopian project based around mere social reform.
Fortunately there is no reason, in principle, why an analogue of Moore's law can't be implemented in successive generations of the reward circuitry of organic life-forms. The affective, aesthetic, intellectual, interpersonal (and spiritual?) well-being of neurochemical robots like us can be genetically pre-coded. If rationally redesigned, our enlightened successors may view today's "natural" rewards as poor surrogates for genetically underwritten happiness. When the mechanisms underlying bliss and its gradients are understood, the molecular machinery of the sublime can be modulated - and amplified indefinitely. Within a few decades at most, we will be scientifically enlightened enough to redesign the neurochemical pathways of emotion. Meanwhile our pleasure centres are too small for us to flourish; and their functional architecture is inefficient. They needn't be either: our normal homeostatic "set-point" of well-being can be genetically ratcheted up to a far higher plane; and archaic Darwinian notions of mental "illness" and "wellness" consigned to oblivion. Gradients of indescribable happiness can potentially animate our lives no less powerfully than gradients of ill-being. Until this fabulous era dawns, then - to borrow the words of Oscar Wilde - "we are all in the gutter, but some of us are looking at the stars".
Critically, such gradients of celestial bliss can also be lucid, serene, entactogenic and empathetic - i.e. MDMA-like and better, not manic or vulgarly hedonistic. The godlike powers of tomorrow's biotechnologists will allow the neurological substrates of empathy and self-insight to be permanently up-regulated. Aesthetically, the mundane ugliness of life in the present epoch can be replaced by gradations of hitherto unimaginable beauty. Potentially again, an E-like magic can imbue the texture of normal waking consciousness. If we so wish, our emotional palette can be genetically enriched, mixed and then pharmacologically refined in ways that transcend the crude primary colours of our Darwinian past.
Counter-intuitively, yet indispensably for the long-term evolutionary stability of a ecstatic society of redesigned post-humans, allelic combinations that promote blissful empathy can also potentially be fitness-enhancing - in the technical Darwinian as well as in the popular sense of "fitness". The dawning reproductive era of "designer-babies" promises to be empowering because the capacity for parental love and nurture can be genetically and pharmacologically enhanced, not just levels of personal happiness, health and superintelligence. The age-old scourge of child-neglect (and worse) can be relegated to evolutionary history. Very speculatively, our future offspring may not merely be more loved by their caregivers, but much more "loveable" too. For if given the [genetic] freedom to choose, then parents-to-be may understandably want their offspring to be loving as well as smart and happy.
The prospect of unleashing such parental freedom is disturbing to most of us. Why not leave babymaking, as before, either to the mysterious workings of Providence or the blind shufflings of selfish DNA? Yet now we're imminently free to choose, there is nothing self-evidently morally admirable about playing genetic Russian-roulette with the lifeforms we create. Many of the nastier behaviours and modes of consciousness that so often proved fitness-enhancing in the ancestral environment will cease to be adaptive if the alleles that promote them tend to be shunned by prospective parents intent on creating the children of their dreams. The "nastier" alleles may well get out-competed. Selection pressure will tend to favour a very different range of heritable adaptive traits once evolution is no longer "blind" i.e. when genotypes are parentally chosen or designed in anticipation of their likely effects on a child's behavioural phenotype. If we want to, we can systematically redesign ourselves and choose the traits of our offspring. The details, for sure, are sketchy. Reproductive science and genetic engineering are in their infancy. But Homo sapiens is poised to bootstrap its way out of the cruel Darwinian abyss.
Inevitably, talk of treating humans like organic robots, and then mooting a baseline of mental health orders of magnitude richer than the Darwinian mind can contemplate, sounds fantastical today. In the context of our traditional conceptual framework, the idea of an analogue of Moore's law for successive generations of human mental health evokes thoughts of cloud-cuckoo-land, not a global health-plan. Set against the daily messiness of our ecstasy-impoverished lives, the prospect of using biotechnology to abolish suffering, and a post-Darwinian transition to paradise-engineering, strikes most of us as fanciful, its liberatory potential just a mirage. At best, such heady words fall lifelessly off the page or screen. Yet a major discontinuity - a momentous evolutionary transition in the development of life on earth - is imminent as the biotechnology revolution unfolds. The advent of genomic medicine is set to challenge the old Darwinian regime of natural selection and the emotionally crippled minds it spawned.
In the long run, genomic medicine can underwrite mental and physical superhealth for everyone. For in principle, lifelong well-being can be genetically hardwired from conception. In the short run, better-designed research tools and therapeutic agents can probe, and then repair, our damaged minds. As chemical stopgaps go, MDMA is a magical revelation. It's perhaps the best aid to insight-oriented psychotherapy ever synthesized. Tragically, when MDMA is used to excess the outcome can be harmful, not healing. So as a weekend club drug, MDMA is seriously flawed. Today, of course, empathogens and entactogens are outlawed for any purpose. The altered states of consciousness they induce are criminalised. People who take such agents are stigmatised as "drug abusers". Yet some MDMA users feel, rightly or wrongly, they've been granted a tantalising glimpse of what true mental health may be like in centuries to come; and an insight into what the rest of us are missing.
David Pearce
(last updated 2023)* * * 2023 Update.
Safe, sustainable analogues of MDMA do not yet exist. The prospect of gene therapy to enable existing human and nonhuman animals to enjoy perpetual MDMA-like consciousness is still decades away. Likewise, routine access of all prospective parents to preimplantation genetic screening, counselling and gene-editing to enable innate MDMA-like mental health as a default condition of everyday life can seem like science-fiction in the wake of the He Jiankui affair and the public backlash against "designer babies". Reflex invocation of the "e" word still retards progress towards genome reform and a reproductive revolution.However, three encouraging developments are worth noting.
First, MAPS' Phase 3 clinical trial of MDMA-assisted therapy for PTSD, published on May 10, 2021, in Nature Medicine, showed statistically significant improvement. In 2017, the FDA granted Breakthrough Therapy designation for MDMA-Assisted therapy for PTSD (cf. MDMA-assisted Psychotherapy (Wikipedia)). The positive MAPS trial augurs well for FDA approval of MDMA-assisted therapy in late 2023 or in 2024. The media routinely describe MDMA as a "psychedelic"; but the distinction between psychedelics and entactogen-empathogens is worth preserving. Putative "psychedelic therapy" for dark, Darwinian minds may be too dangerous and unpredictable. MDMA is different.
Second, PharmAla Biotech's MDXX drug discovery program of MDMA analogues delivered its lead drug candidate, ALA-002. Information on the subjective effects of ALA-002 is scanty even within the scientific counter-culture. In November 2022, PharmAla announced it had submitted its pre-IND data meeting package on ALA-002 to the FDA. PharmAla hopes that ALA-002 can be used in the treatment of adults diagnosed with Autism Spectrum Disorders, broadly defined. The Canadian biotech company researches and develops Novel Chemical Entities (NCEs) based on the MDXX class of molecules with a view to improving their safety and efficacy. The base MDMA molecule can be improved upon. If PharmAla Biotech succeeds in its mission, Utopian Pharmacology can become more than an empty label.
Third, a revolution in AI heralds a passable imitation of artificial general intelligence. GPT-4 is a gamechanger. Digital zombies can now write more intelligently about consciousness and MDMA than the vast majority of humans. For example, see ChatGPT-4 on MDMA. In collaboration with human investigators, GPT-4 and its cousins can play a role in psychoactive drug discovery and novel MDMA-analogues in a recursive cycle of improvement. To be clear, classical Turing machines and connectionist systems are idiots savants whose insentience is architecturally hardwired. "Digital mind" is an oxymoron. Phenomenal binding into mind is classically impossible. So classical computers are not going to "wake up" and become psychonauts - i.e. phenomenally-bound subjects of experience like humans who explore myriad state-spaces of consciousness. In consequence, full-spectrum superintelligence - supersentience, so to speak - will most likely retain a neuronal core. But AI plus psychonautic research promise an awesome future for sentience in the cosmos.
2024 Update.
In June 2024, a study published in the Journal of Neurochemistry identifies three new variants of MDMA as promising candidates for safer use: ODMA, TDMA and SeDMA. The three drugs have been developed with a view to retaining the extraordinary positive effects of taking MDMA while reducing adverse after-effects. The authors write: “Our findings suggest that these new MDMA bioisosteres might constitute appealing therapeutic alternatives to MDMA, sparing the primary pharmacological activity at hSERT, hDAT, and hNET, but displaying a reduced activity at 5-HT2A/2B/2C receptors and alternative hepatic metabolism. Whether these MDMA bioisosteres may pose lower risk alternatives to the clinically re-emerging MDMA warrants further studies."Also in June 2024, a U.S. Food and Drug Administration advisory committee voted that evidence is lacking that MDMA-assisted therapy is effective for treating post-traumatic stress disorder (PTSD). The advisory committee reported that the benefits of MDMA-assisted therapy don’t outweigh the risks to patients. Panellists voiced various concerns with the data. Issues ranged from study design; uncertainty whether MDMA-assisted therapy offers long-lasting benefits; and the potential risk of heart problems and abuse. The sponsor of the MDMA treatment, Lykos Therapeutics, formerly MAPS, conducted two clinical trials showing that patients treated with MDMA experienced a significant improvement in their PTSD symptoms relative to subjects who received only a placebo. Unfortunately, the consciousness-altering properties of MDMA also vastly complicate the double-blind, placebo-controlled gold-standard for clinical trials. An FDA official, Tiffany R. Farchione, told the panel that although the agency had asked Lykos to report impacts associated with abuse, Lykos did not note effects such as “euphoria” or “elated mood.”
On August 9th, the FDA accepted the advisory committee's recommendation and declined to approve MDMA-assisted therapy for PTSD. In its letter of rejection, the FDA asked the drugmaker Lykos Therapeutics further to study the safety and efficacy of the proposed treatment. The decision was widely reckoned a setback for the nascent field of psychedelic medicine. But classifying MDMA as a psychedelic in the first instance is problematic. Not least, psychedelics don't tend to promote a heightened sense of authenticity: this-is-the-real-me. MDMA is in a class of its own.
Research on safe and sustainable MDMA analogues continues. Research on safe and sustainable MDMA analogues continues. See ODMA, TDMA, SeDMA (Wikipedia). But the vision of loved-up life and civilization sketched in Utopian Pharmacology (2002, 2024) is currently a distant dream.
dave@hedweb.com
Refs
and further readingHOME
HedWeb
2015: talks
MDMA hotlinks
Claudio Naranjo
MDMA synthesis
Superhappiness?
Alexander Shulgin
ChatGPT on MDMA
Accidental Ecstasy?
Social Media (2024)
The Good Drug Guide
Ecstasy and Honesty
The Abolitionist Project
Quora Answers (2015-24)
The Reproductive Revolution
Critique of Brave New World